 |
Office of Media Relations | |||
News | ||||
|
New Class of Compounds Promise Better Drugs, Clean Energy
Brown University chemists have created a new class of compounds that promise to produce prescription drugs more cheaply as well as to provide models for hydrogen storage – a key feature for clean energy production and use. The work has landed in top journals, including a cover of Chemical Communications this month, and has prompted two patent filings. PROVIDENCE, R.I. — By combining a common organic compound with a rare metal, a team of Brown University chemists has created a new class of molecules that have potentially important applications for the pharmaceutical, chemical and energy industries. To create the mixture, scientists working in the laboratory of Dwight Sweigart, a Brown professor of chemistry, combined two compounds. One is hydroquinone, pale organic crystals critical for many biological processes as well as the manufacture of everything from skin bleaching creams to high-performance plastics. The other is the precious metal rhodium. The resulting reaction produced rhodium quinones. 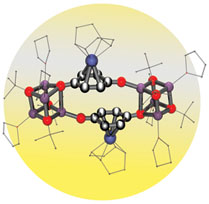 New possibilities for catalysis, synthesis, storage “This mixture has marvelous properties,” Sweigart said. “Rhodium quinones are very fast and efficient catalysts. They also have pores, or channels, that act like a sponge, giving them the ability to store gases. The secret is rhodium. It’s the Superman of elements.” Rhodium is lighter than platinum, rarer than gold, and, at about $3,000 an ounce, the priciest of precious metals. The silvery white substance is prized as a potent, long-lasting catalyst and is used to concoct antifreeze, detergents and other industrial chemicals as well to make automotive catalytic converters, which cut down on air pollution. Rhodium is also the most reflective element on the periodic table and can be found in searchlights, dental mirrors, and giant microscopes known as synchotrons. Discovery of rhodium quinones has landed Sweigart and his research team in premier chemistry journals, including the Journal of the American Chemical Society and Angewandte Chemie, the publication of the German Chemical Society, which put the compounds on its December cover. This month, the research landed on another cover: the British journal Chemical Communications. Together, the articles outline potential applications of rhodium quinones:
“After routinely working until 2 or 3 a.m. in the lab, creating the new compound is extremely exciting,” said Jeffrey Reingold, a graduate student working in the Sweigart lab. “The rhodium and the quinone parts of the molecule each contribute unique characteristics to generate a powerful new reagent with enormous potential. Much of our future research will focus on the development of this fascinating chemistry.” The research team also includes chemistry graduate student Sang Bok Kim, professor emeritus of chemistry Gene Carpenter, and Seung Uk Son, a former Brown post-doctoral research fellow and current assistant professor of chemistry at Sungkyunkwan University. The National Science Foundation and the Petroleum Research Fund funded the work. ###### Media Relations Home | Top of File | e-Subscribe | Brown Home Page | ||||