
|
May 2, 2007 |
Liquid CO2 Drives Rapid Thrust of Diamond-Bearing Structures
In the May 3 issue of Nature, James Head, a Brown University professor of geology and Lionel Wilson, a professor of volcanology at the University of Lancaster, propose an integrated and dramatic mechanism for the formation of kimberlites, the enigmatic structures bearing most of the world’s diamonds. Their theory explains many puzzling features of the formations and also suggests that the location of kimberlites is not related to near-surface geology. | |||
|
Brown University Home |
PROVIDENCE, R.I. [Brown University] — Freeze-dried ice cream looks like the original product, and even tastes pretty good, but “drying” ice cream at room temperature would leave a sour-smelling, sloppy mess. Similarly, diamonds ejected from deep in the Earth can survive the journey intact only if they head toward the surface quickly and under just the right conditions. Researchers at Brown University and the University of Lancaster have a new theory to explain how diamonds could make it to the surface. 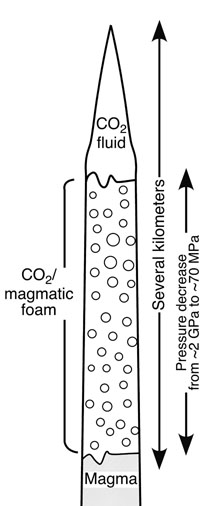 A geological mechanism for diamond transport Diamonds, found almost exclusively within kimberlite formations, are stable at great depth and at the planet’s surface but rapidly turn to graphite under the high temperature/low pressure conditions of most eruptions. A new integrated theory of kimberlite formation, published in the May 3 issue of the journal Nature, invokes a leading wedge of fluid carbon dioxide to explain how the diamonds survive as magma powers them upward. When the carbon dioxide breaks through the surface, the sudden pressure change causes the liquid to expand rapidly to gas, flash-freezing the magma and stopping the eruption in place. “As long as [the diamonds] are hot, you want them to stay at high pressure,” said Lionel Wilson, a professor of volcanology at the University of Lancaster and co-author of the Nature paper, “and then if you’re going to decompress them, you’ve got to get them cold really fast.” Wilson and James W. Head III, a professor of planetary geology at Brown University, developed the theory while searching for a solution to the problem of how round glass droplets might be formed on the moon where there is no atmosphere and little gravity to shape them. As the scientists talked over one proposed mechanism, recalls Head, they each thought, “Hmm, that could explain how kimberlites form.” “We had tried to understand how you get this glass that comes out of eruptions on the moon,” said Head. “The chemistry we saw seemed to require low pressures at great depth, which is kind of an oxymoron. We realized the molten rock comes up in a crack and when the crack opens, there is a small zone of very low pressure.” A handful of nagging questions have challenged geologists trying to explain the genesis of carrot-shaped kimberlite formations. The diamonds are one problem. The shape is another. The inverted cone of a kimberlite is filled with cracked and broken chunks of solidified magma, mixed with glass globules and some diamonds, all confined in a narrow, underground space, not scattered across the landscape as they would be in a normal volcanic explosion. Geologists find little evidence for flowing lava around the formations, suggesting that the eruption is suddenly capped or stoppered. Also, glass globules, like the moon artifacts Head and Wilson were trying to explain, appear throughout the formation and it’s not at all clear how they could be formed underground. In the mechanism that Wilson and Head suggest, a wedge of liquid carbon dioxide forms above a source of carbon dioxide-rich magma at a depth of about 250 km. As the wedge drives upward, it fractures the rock it passes through, dropping fragments into the tube of magma beneath it. Streaming upward at speeds of 100 to 180 kilometers per hour, the underlying magma resupplies the carbon dioxide in the tip chamber. The column forms into three segments: magma on the bottom, fluid carbon dioxide on top, and a layer of magma foam in between. The pressure driving the magma is nearly 30 times greater than at the tip of the column and the difference draws carbon dioxide out of the magma, forming a head of foam. Head and Wilson think this frothy mix of magma and carbon dioxide is the source of the unexplained glass spheres. When the tip of the carbon dioxide chamber breaks the surface, the fluid rapidly expands to become a gas, sending a jet of carbon dioxide, magma foam, and rock fragments shooting into the air at speeds of up to 5,000 km per hour, typical of a booster rocket or a jet engine. A cavity three kilometers deep might empty in only 10 seconds. This rapid expansion flash-freezes the magma near the surface and sends shock waves through the cavity, imploding the cavity walls and filling the chamber with rubble. Pressure builds once again in the mostly sealed chamber and waves of expansion and compression bounce up and down the chamber, cooling and shattering magma, breaking up the surrounding rock, spattering magma droplets into glassy spheres and sorting and rearranging the resulting fragments. The rapid chilling soon seals off the magma supply completely, ending the process. The whole progression likely takes less than an hour. One implication of the new theory is that surface geologic conditions have little to do with where kimberlites form, and thus where diamonds are deposited. Unlike some recent theories of kimberlite formation, no source of underground water is needed to drive the explosive power of the formation. If the theory proves true, it would suggest that diamonds have been found in South Africa or on the Canadian Shield mainly because that’s where people have looked for them and that a concerted search could turn up kimberlites – and the associated diamonds – anywhere. This work was supported in part by a grant from the National Aeronautics and Space Administration. Editors: Brown University has a fiber link television studio available for domestic and international live and taped interviews and maintains an ISDN line for radio interviews. For more information, call the Office of Media Relations at (401) 863-2476. ###### | |||