1. Visual acuity: observe a 125 micron tungsten rod, against white background.
visible at a distance of say 12.5 meters:
125 microns = 125 x 10-6 meters
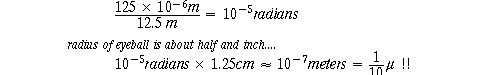
Rods, Cones and Phototransduction: Notes 4/03 , 4/05
Goals:
¶ Be able to explain the negative-going voltage of rods, in response to
light.
¶ Understand what is responsible for the long latency of a rod response
¶ Understand why dark adaptation takes so long (~hour).
¶ See how G-protein transducin, enzyme PDE6 and cyclic nucleotide cGMP
(cyclic adenosine monophosphate) combine to affect sodium channels in rod outer
segment membrane.
Simulation: See Assgn A: modelling the concentration of [cGMP] and rod
intracellular voltage. Theme of assgn A: why does it take 100 msec for the rod
response to develop?
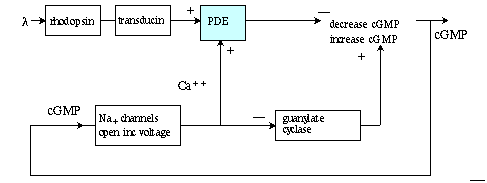
Understanding reactions from rhodopsin to cyclic GMP: Quick summary:
Rhodopsin changes conformation to R* which cleaves G protein
Transducin whose action uninhibits enzyme PDE6, which converts cGMP to 5'GMP.
Cyclic GMP keeps membrane sodium channels open so its absence causes channels
to close. Sodium has a positive equilibirium potential so the rod intracellular
moves closer to the negative potassium equilibrium.
Reading
Lubert Stryer, "The Molecules of Visual Excitation", Scientific
American, July 1987, pp 42-50
Denis Baylor & Julie Schnapf, "How photoreceptors respond to light,"
Sci. Am., Apr. 1987, pp. 40-47.
John Dowling, The Retina, Harvard Univ Press, 1987. QP479/D65. see chpt 7, "Adaptation and photoreceptor mechanisms".
Tom Cornsweet, Visual Perception, Academic Press (1970), chpt V.
Demos:
1. Visual acuity: observe a 125 micron tungsten rod, against white background.
visible at a distance of say 12.5 meters:
125 microns = 125 x 10-6 meters
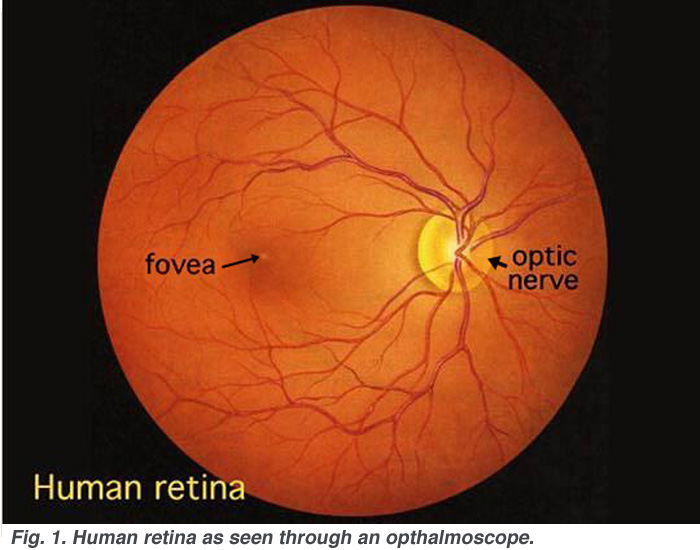
2. OPHTHALMASCOPE for visualizing retina.
the first sensory system we can see!
look for blood vessels on the retina, blind spot, fovea.
Image below from http://webvision.med.utah.edu/imageswv/huretina.jpeg
Note color!
hyperacuity
rods vs cones--wavelength sensitivity
seeing UV through an artificial lens.
density of photoreceptors--fovea: all red and green cones.
Head rotation causes mechanical bending of photoreceptors, like the hair cells that were bent...
dark current of retina: forms a "battery" that is the basis for EOG method of eye movement monitoring.
Sensitivity: Rods are sensitive to one photon (see recordings from Baylor, Nunn & Schnapf, "The photocurrent, noise and spectral sensitivity of rods of the monkey macaca fasicularis," J. Physiol. 357: 575-607, 1984). A human can detect as few as 6 photons at a time. Rods have a response range of 3 orders of magnitude, up to 10,000 photons/sec (mesopic vision), at which point they saturate. When rods saturate cones start responding (photopic vision), and by shifting their operating curves, cones take the human eye out to 10 billion photons/sec: noon sun on freshly fallen snow at altitude. 9 orders of magnitude!
Structure:
photoreceptors at back of retina! light must travel through all other cells...transparent
as they are.
in cats the retina is near tapetum, for reflection of photons back through receptors.
Cornsweet book, page 357, cone spacing, 3 microns. Rods about 5x larger than
cones.
The disks of the outer segments: About 2000 of them. In their membranes is
rhodopsin protein.
Do the disks hold the second messenger? no: cGMP is not sequestered.
Sodium channel proteins: 1 for every 500 rhodopsin molecules. (Applebury review).
Nature News & Views April 9, 1987, page 546, Meredithe L. Applebury.
"Biochemical puzzles about the cyclic-GMP-dependent channel."
G protein in phototransduction
Transducin is one of many G-proteins, which are discussed in Sci. Am
July 1992 page 56 (Alfred Gilman) and Nature 357: 563 June 18,
1992. (Gustaducin) Nobel prize for Alfred
Gilman in 1994. Gilman notes that another G protein affects smooth muscle
cells in blood vessels (Gq) and still another affects cardiac muscle. G protein
regulates production of a "second messenger," in the case of photoreceptors,
of cyclic GMP (guanosine monophosphate). "The [G protein] field did not
truly blossom, however, until 1984, when the genes for the first members of
the family were cloned."
From Rhodopsin to sodium channel: the phototransduction process (Lubert
Stryer paper)
see copy of figure on page 49 as captured image...
In the dark cGMP keeps Na+ channels open in rod membrane.
photon absorbed by 11-cis-retinal causes conformation change to trans-retinal.
activated rhodopsin catalyzes the conversion of GDP to GTP in transducin protein
alpha subunit.
The activated alpha breaks away from beta and gamma subunits.
alpha pulls inhibitor away from PDE-6 (phosphodiesterase).
PDE6 converts cGMP to 5'GMP.
Reduction cGMP concentration closes Na channels.
Cell hyperpolarizes
Where is the gain in the cascade?
Two places:
ONE: Stryer: "When rhodopsin [the chromaphore retinal, to be precise] was
activated by light, we found that each molecule of rhodopsin catalyzed the uptake
of 71 molecules of a GTP analogue to the membrane. Thus it appears that each
molecule of rhodopsin was activating transducin by catalyzing the exchange of
GTP for GDP in many molecues of transducin." [let's say 500] and "The
production of hundreds of active alpha-transducin GTP complexes by a single
rhodopsin is the first stage of amplification."
This effect of R* on transducin would be a serial activation, one transducin
after another.
It also appears that it is a metastable state, metarhodopsin II, that
is responsible for the release-and-activation of transducin, and that this state
lasts about 10 msec; therefore the rate of activation could be seen as 50000/sec.
TWO: "The departure of gamma [on PDE] unleashes the catalytic activity
of the phosphodiesterase. That activity is powerful: each activated enzyme molecule
can hydrolyze 4200 molecules of cyclic GMP per second." [Dowling
says 2000/sec; 500 x 2000 = 1M gain]
What stops (and reverses) the the process? "The alpha subunit [of transducin,
the part that uninhibits PDE6] has a built-in chemical timer that terminates
the activated state by converting the bound GTP [back] to GDP. The mechanism
of the timer is not fully understood."
Rhodopsin conformation (R*) active state is brief; soon the activated chromaphore
(all-trans retinal) detaches from the opsin protein. How an 11-cis retinal reattaches
itself to the opsin is a "long" story.
The phosphodiesterase enzyme in the retina
is one of several types in the body, and is numbered PDE6. PDE3 for example,
is involved in the control of cardiac contraction. PDE5 is the phosphodiesterase
inhibited by sildenafil (viagra). Sexual stimulation leads to the release
of nitric oxide (NO) which activates the enzyme guanylate cyclase, an
enzyme that helps produce more cGMP. The chemical cGMP is involved in the relaxation
of smooth muscle associated with increased arterial blood flow into the penis,
thereby enabling erection. Viagra inhibits PDE5; an enzyme that "degrades"
cGMP, thereby interfering with erection. Thus the effect of viagra in conjunction
with nitrous oxide NO release is to maintain penile erection.
Viagra has only a 10:1 selectivity for PDE5 over the retinal PDE6. Thus, with high doses of Viagra, visual disturbances can be expected, and turn out to be confusion with green/blue distinction. Airline pilots who have taken Viagra are not allowed by the FAA to fly until the drug wears off: regulations call for a 12 hour wait.
Rod saturation, rhodopsin bleaching and recovery. Why does a rod saturate? Are all the rhodopsin molecules converted to the trans form? No, there is so much gain in the phototransduction process that rod saturation occurs because sodium channels are nearly all closed. Note that there about 100 million rods, about 1000 disks per rod, and about 1 million rhodopsin molecules per disk, for a total of 10^17 R molecules in the retina. Average distance between R's: 20 nm = 200 Angstroms. If rods saturate at 10,000 photons/sec, then in 10,000 seconds only 10^8 out of 10^17 R's are struck by photons. Even if the light is 10 billion photons/sec, only 10^14 R's will be converted in 3 hours! A bleaching stimulus can be much brighter (1000x) than 10 billion photons/sec.
Rhodopsin converted to the all-trans conformation is sometimes said to be bleached. Bleaching refers to most of the photopigment molecules in the retina being inactivated by a bright light. In fact the retina after exposure to bright light does look bleached (see Dowling book, The Retina, color plate 7, opp. p. 83). What is the process of regenerating 11-cis retinal?
Adapted from Dowling, The Retina, chpt 7, p. 191: Following exposure to dim adapting stimulus that did not bleach measurable ... pigment, dark adaptation was v. rapid ... completed in less than a minute. After an intense bleaching light ... the extent of slow phase adaptation was related to the amount of pigment bleached. ...slow phase adaptation is photochemical adaptation.
From Cornsweet (1970) chpt 5: p. 94-95: A v. bright 1 msec flash of light bleaches half the rhodopsin. Another flash bleaches half of the remaining. During the flash repeated photon capture sends rhodopsin flip-flopping between 11-cis and the next state of retinal. The full transition from 11-cis to all-trans can have a time constant of 1000 msec! [There is the delay of phototransduction.]
Dowling: The light sensitivity of the visual pigments is due to a chromophore ... derived from vitamin A, called retinal ... which is bound to the protein, opsin. It is retinal that changes from 11-cis to all-trans form (Dowling, Fig 7.5) but the opsin molecule is also affected. If the chromophore separates from the opsin, then bleaching has occured. In continuous bright light most of the released retinal is reduced to vitamin A and stored in the pigment epithelium [region beyond the photoreceptors, in which the photoreceptors are embedded]. Dark adaptation requires the converting of vitamin A into 11-cis retinal, which will spontaneously attach to bleached opsin. See Fig 7.8 of Dowling.
p. 202: It has long been known that an isolated retina does not regenerate rhodopsin. The [rod] outer segments must be in close contact with pigment epithelium for the isomerization of all-trans to 11-cis retinal to occur. It is possible to induce regeneration by adding 11-cis retinal directly to the isolated retina (!).
In the isolated retina a linear relationship between log threshold sensitivity to light and pigment bleached can be seen.
In the intact eye visual sensitivity can be decreased by withholding vitamin A from the diet of a human subject.
Role of Calcium
McNaughton reprint: Ca++ internal concentration may mediate light adaptation...
Assume Ca++ can leak through the sodium channel.
light adaptation implies reduced sensitivity (to greater light levels). Therefore
the process needs some chemical to mimic the effects of light...adaptation may
be a function of Calcium: see Nature 334 July 7, 1988 review:
Calcium activates PDE and thereby decreases cGMP
levels, but [Ca]i is reduced in the light, so cGMP increases, closes channels...the
opposite effect of photons: negative feedback...
TINS Sept 1990 page 378, Gordon Fain, "Calcium and and the mechanism of light adaptation in vertebrate photoreceptors." "The probability of channel open in darkness is 1-2%."
PDE lowers level of cGMP. Ca++ also activates PDE and can therefore keep cGMP levels lower. In the dark Ca++ enters and equlibrates cGMP. In the light less Ca++ can enter and therefore PDE levels are reduced a little, increasing cGMP.
Feedback and the role of calcium: See Assgn A.

Cones
How are cones different from rods?
outer segments open to extracellular space.
smaller diameter.
higher threshold for activation
higher level of saturation, adjustment to background illumination.
comes in three varieties, with the blue cone most likely to be mutant.
color blindness only in males.
Brown University, April 4, 2003, lecture by Julie Schnaph,
UCSF, "Receptive fields of cone photoreceptors in macaque retina"
intracellular recording from cones, on inverted
retina, pigment epithelium stripped.
role of horizontal cell feedback in affecting chloride channel conductance of
cones.