Lab LTL / LTA: Frog Leg Muscle Length-Tension Curve
There are
two ways to do Lab LT:
(1) Lab LTA--program a motor-driven stage for automatically moving a frog with
respect to a force transducer attached to its Achilles tendon: from your VI
automatically send a trigger signal to the nerve stimulator, then record the
resulting twitch sensed by a force transducer, capturing the max and min at
each 0.5 mm interval in an array, advance the stage by your VI controlling a
stepper motor driver, let the muscle rest for 5 seconds, then automatically
repeat the process until the muscle is stretched to its limit. Have then array
of data saved, and likely sent to EXCEL for further processing.
OR,
Lab LTLight = LTL--for fewer points, for those wanting to pass 123 or qualify
for a B, or being so comfortably in the A-zone LTL is all that's needed...
The only VI you'll need to create will have one frame waiting for a trigger-out
signal from the nerve stimulator... when that signal is received the VI will
have a second frame collect force transducer data for 1 second and determine
max and min force in that interval. You will enter those numbers in a spreadsheet,
then manually advance the frog tray 1mm to stretch the muscle for another twitch.
Repeat until the force transducer saturates or the twitch disappears.
In either
case there are
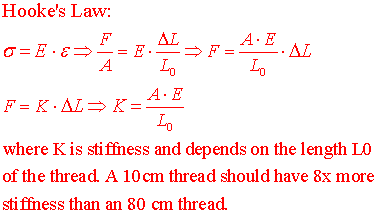
calibration measurements to be made in 095, for the stiffness K of the surgical
thread, and the conversion of transducer volts to newtons.
You will generate the length-tension curve of passive and active gastrocnemius muscle from 2.5" small rana pipiens, the leopard frog.
Background: The passive
elastic properties of a muscle can be modeled as a nonlinear spring. The force-tension
curve becomes exponentially steeper at longer lengths of stretch. ![]() where σ is stress and ε is strain. Solve for σ !
where σ is stress and ε is strain. Solve for σ !
When muscle is held to a constant length then stimulated by way of it's nerve connection from the ventral spinal cord, the muscle develops additional tension beyond passive. This active force is derived from interactions of the actin and myosin protein filaments. The active force length tension curve is described by a racheting filament model and has its maximum near the muscle's normal rest length. At this length is the most interaction between the actin and myosin filaments, accounting for the largest active force production. At shorter or longer lengths the filaments have less overlap to form crossbridges; they therefore produce less force.
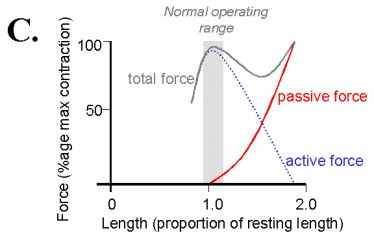
On the diagram above the distance
between the total force and passive force curve is the active force.
Requirements: (stated for
LTA)
(0) The (planetary gear + stepping-motor)-driven tray carrying the anaesthetized
frog will be moved by commands from LabVIEW. The motor requires +22v/1Amp of
power and needs 3 control signals: ON-OFF (active LO, DIRection (CW or CCW),
and clock (determines the speed of rotation). Thanks to the planetary gear (14:1)
in the OFF state the gears will prevent the motor from slipping.
The stage has an M6 lead screw (1mm per pitch)attached by a coupling to the motor armature.
Clock for the stepping motor driver can be supplied from an external function generator (0-4v p-p square waves at about 7KHz) or a AD654 VCO. (See ADA Lab diagram for 654 pinout and external R's and C...)
(1) At the end of the "pull" direction path of the motor is a lever switch whose output is normally HI but will go LO if the switch is hit by the stage. If your software senses a LOW from the limit switch it will reverse the motor and run the tray off the switch for about 1 second. The experimental part of Lab LT will be over.
(1b) You will need to accommodate control signals DIR, ON-OFF, and Stim Trigger. One way: use a DAC output for the trigger and two digital outputs for DIR and ON-OFF.
(2) Using a mm ruler you will want to determine (by running the stage for say 5 mm) how many seconds and how many motor armature rotations correspond to 1 mm of linear movement.
(3) Fixed with respect to the base holding the tray stage and motor will be a force transducer with a length of surgical thread connecting the force transducer (500 gm max reading...) to the Achilles tendon of the gastrocnemius muscle under study. The output from the force transducer will go to an Ain channel of LV and be read by your VI for one second. The VI will determine the minimum (passive) and maximum (active + passive) response due to a twitch of the muscle. That data will be accumulated as a function of stretch (cause by the motor moving the stage).
(4) Electrodes under the sciatic nerve of the frog will be stimulated and the resulting shock will cause the gastrocnemius to twitch. Your VI should have an output signal that triggers a stimulator to send out the pulse twitch.
(5) Once the frog is set up,
and your VI starts, it will
* Record the current voltage from the force transducer
* Send out a 5 msec trigger *pulse* to stimulate the muscle for a twitch
* Record 1 second worth of force transducer output
* Save the max voltage seen from the 1 second worth of twitch response
* wait 5 seconds (muscle recovery)
* automatically pull the tray 0.5 mm away from the force transducer
* repeat the process until the limit switch is hit or the force transducer saturates
or the twitch disappears. About 40 data points will be taken.
DATA ANALYSIS:
* Generate an ACTIVE column of max-min
* on your spreadsheet, create a new column of
"normalized passive" = min-(first passive reading) so the first data
point is 0
* Convert volts to newtons: about 4 volts per 200gm of "force" is
a reasonable calculation...
* Plot as a function of mm stretch : norm passive tension, and ACTIVE tension
(6) The process is not repeatable
because the pulling on the muscle stretches it plastically beyond its normal
limits of play. If needed, you can obtain a second set of data by using the
left leg...
Create your VI before going over to Arnold Lab...convert your VI to LabVIEW 7.0
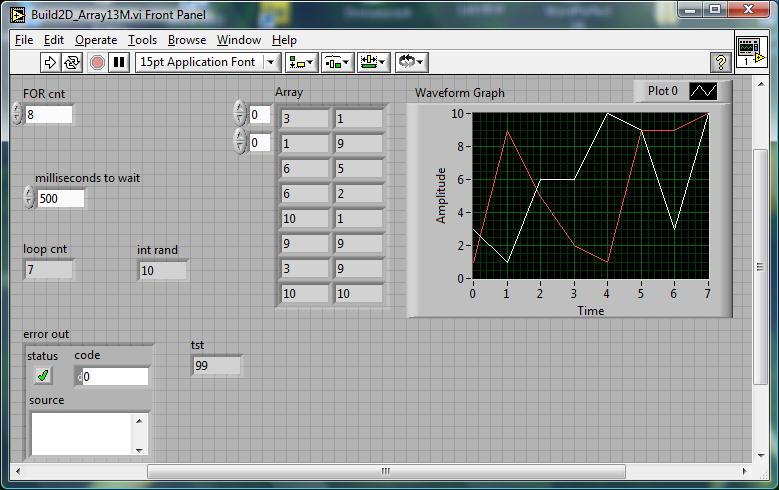
Below--an example VI that
looks for max and min in a 1 second interval...
FIGURE 1
MIN will be the passive tension, and MAX will be active+passive tension, for a fixed length of muscle (isometric).
Use "Save with options" for your VI created in B&H, to convert it a 7.0 version (not our 7.1); 7.0 is the version machines in Arnold B-20 Neuro 160 use. The conversion will create a new folder with the same VI name...your 7.0 VI is inside that folder...
(7) CALIBRATION: In 095 there will be a ring stand with a MLT500 mounted and attached to a 200 gm weight. The MLT500 is connected to a gain-of-1000 AD524 Inst. Amp, and the multimeter is connected to the output of the 524: Note how much the voltage on the changes when the weight is lifted. You will need the information to translate voltage into tension and to calculate the stiffness of passive muscle.
(8) After you return from B-20 look for the approx 100 cm length of green surgical thread (that will be in series with the gastrocnemius muscle) attached to a 1Kg + 100 gm tray. Lift the 1100 gm weight with the thread and measure the ΔL increase in thread length. What will be the stiffness of a 20 cm length of thread that attaches from the tendon to the MLT500 in B-20? See formulas below.
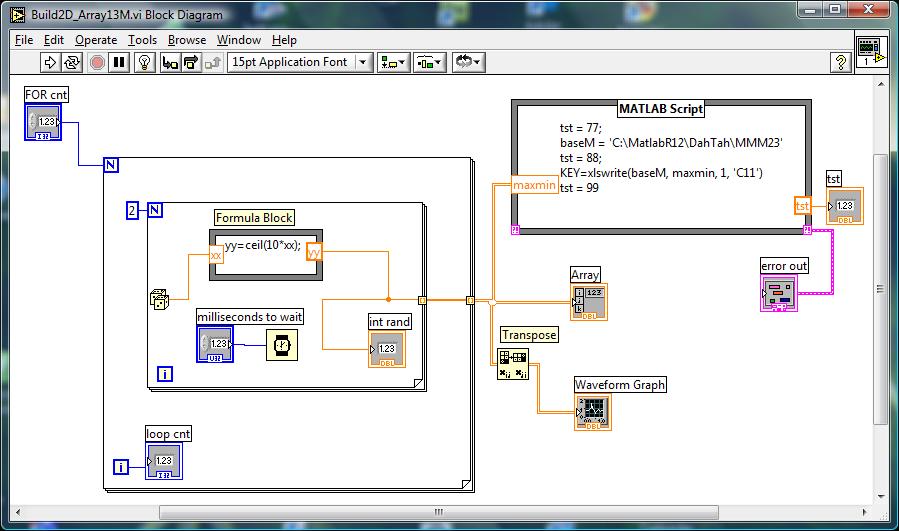
Idea for saving data accumulated in LTA min-max loop:
where the dice loop represents your collection
of min and max on each of the FOR loops representing stretch of the muscle...
Below--additional information about Lab LT:
(1) Dissection for
gastrocnemius muscle and sciatic nerve:
We'll use rana pipiens, the leopard frog. You are welcome to help with
the dissection (esp if you are premed), or we can do all physiological preparations
for your team:
Pith CNS, and pith down the spinal cord to eliminate unwanted spinal reflexes. Before pithing test withdrawal reflexes of frog for before-and-after comparison. Write down the time of pithing. The frog may give useful responses for up to 2 hours after pithing. Pithing: Insert a 10 cm rigid probe (tapered to a sharp point) through the skin into the foramen magnum between the base of the skull and the top of the vertebrae. (midline, at the level of the caudal tympanic ovals. Pull out and reverse direction of probe to pith spinal cord.
link
to dissection of gastrocnemius
¶Remove skin from leg: Sous chef technique
¶Blunt dissection of "calf" muscle: sew 2-0 silk through beginning
of distal tendon. Tie square knot at the tendon. Remaining 2-0 (about 10 cm
each length) to be tied in a loop for attachment to force transducer. Cut tendon
away from bone at a more distal site.
¶Blunt dissection to reveal sciatic nerve from abdominal view. Note sex
of frog (presence of egg sack = female...) Try not to nick open the thoracic
cavity, so the lungs don't collapse. Be mechanically gentle with sciatic nerve.
Pull 2-0 silk thread under the nerve to help lift over stimulating electrodes.
Use Ringer's solution for
exposed tissue. Also known as Ringer’s irrigation: specifically
for amphibians, in one liter distilled water mix:
6.5 g NaCl
0.42 g KCl
0.25 g CaCl2
Force transducer: We will use the ADInstruments MLT500 force transducer,
which can handle up to 500 gm wt force. See
http://www.adinstruments.com/
for information. The pdf link below tells you what color wire goes with which function
on the MLT500. http://www.adinstruments.com/products/datasheets/MLT050.pdf
We will excite the MLT500 bridge from a fixed-voltage triple-output supply. Notice that the data sheets in the pdf file say that 10v is the MAXIMUM excitation voltage to be applied for the MLT500! Use the 5v supply for the excitation voltage!
Because both sides of the MLT500 bridge float w.r.t. ground, we want to make a differential measurement sending each side into a nearby 524 instrumentation amp (gain of 1000). That arrangement will be your "preamp" and allow you to go directly into a single-ended scope input AND a LabVIEW Ain channel (BNC connections). No need for rack preamp or output from back of 'scope...
(after the data collection...) calibrate the force transducer by hanging a 200 gm weight from it and writing down the resulting DC voltage. It will be in the mV range! (Consider the Tektronix scope as your amplifier, as you did in Lab 8.) What is the unloaded voltage from the MLT500? We will install one our Agilent DMM's in the setup to read off passive voltage continuously, and to make sure the 524 does not saturate.
Mechanics: The MLT500 uses a Wheatstone bridge of 4 strain gauges, arranged on a cantilevered beam of low stiffness. Note where TOP is on the MLT500; Another way to measure muscle tension is to suspend the muscle vertically. Yet another way: use a pulley to redirect horizontal stretch vertical.
Display the force transducer output on the Agilent scope we will bring from B&H. Trigger the scope from the stimulator. Use DC for recording.
Thread from muscle to force transducer: We don't want to measure the stiffness (or lack thereof) of the thread attached to the muscle. We will use Ethilon #2 braided green surgical thread, which has a good combination of strength, flexibility, friction, and stiffness. We'll tie slip knots with the thread to attach to the transducer.
AM Systems model 2100 stimulator: Examine the controls on the front panel. Begin with a 3 msec duration pulse of 2 mA amplitude, flick output control momentary rocker switch to Manual when a shock is required. Send sync from trigger BNC to EXT on the Agilent scope, and to a second LabVIEW Ain channel.
(5) Mechanical arrangement of gastrocnemius and force transducer: We will drive two dissecting pins into the white silastic base of the dissecting tray, on either side of the proximal tendon, near the frog "knee". Do not push the pins through tissue: the pins will be needed to restrain the frog leg from moving during stretch. We attach the thread loop tied to the distal tendon to the force transducer using a slip knot.
Be sure to measure and record the rest length of your muscle. The length
should be in the range 20-30 mm.
Nerve stimulator: The stimulating electrodes are attached by flexible stranded wire to and electrode holder on the side of the movable tray. Make sure no extraneous tissue or fluid shorts out the stimulation (clear view underneath the raised nerve). Use manual single shocks to watch the leg twitch. If you don't see a strong twitch when you stimulate, check the settings on the stimulator, and check that the sciatic nerve is lifted up and electrically isolated on the stimulating electrodes.
To begin with, we won't care to isolate only the fibers headed for the muscle in quesion--the whole leg will twitch.
Observing twitches on the scope. What is the latency in msec from stimulation to contraction start? contraction max? What is the duration of a twitch?
Slowly increase the stim. from 3msec duration, 0.1mA current, until you observe
maximum twitch tension, then increase the current by another 10% to ensure a
supra-maximal stimulus.
To insure response over the full range, offset the scope to a negative voltage,
so the scope itself doesn't saturate its output. Start with a relaxed setting
in which you see no twitch.
EXTRA: Once you finish verifying your LT curve, cut the sciatic nerve above the electrodes and retest the passive data. Was there any influence on the passive data of a "stretch reflex" mediated by the spinal cord? Or evidence of plastic deformation?
(10) Calculate the maximum passive stiffness of the frog muscle and compare it to your calculation of surgical thread stiffness.
(Archive) possible FTQ's:
1. Explain what would happen if something your VI were altered...
2. In the equation ![]() what do you estimate alpha to be for your passive muscle data?
what do you estimate alpha to be for your passive muscle data?
3. Explain how sliding filament theory accounts for negative L-T curve.
Reading: pages
8-11 from McMahon, Muscles, Reflexes and Locomotion.
figure from McMahon.
Links: frog legs are a delicacy of French (and Chinese) cuisine. see link. Most frog legs these days come from frog farms in Thailand and Indonesia.
What is the longest muscle in the human body?
To B-20:
DMM, function generator
power supply with force transducer & 524 set to gain 1000
oscilloscope
power supply for stepping motor
surgical instruments: pith rod, scissors, clamps, rat's tooth forceps, sharp
tapered forceps, sewing needle
plastic bags
paper towels
In 095
vise for large clamps to hold end of 75 cm surgical thread tied to 1.1Kg weight
on jack stand
power supply with 524 attached to force transducer attached to 200gm weight
on jack stand