Complications of acute M.I. occur in a time-dependent manner, and can be directly related to the anatomy of the coronary artery blood supply. Complications may occur due to ischemic or injured tissue and therefore may begin within 20 minutes of the onset of M.I., when myocardial tissue injury begins. These complications include arrhythmias and heart block (due to injured or ischemic conduction system tissue), and hypotension and congestive heart failure (due to ischemic or injured muscle tissue, resulting in abnormal filling {"diastolic dysfunction"} or abnormal emptying {"systolic dysfunction"}).
Several days later, complications of M.I. can ensue due to "yellow softening" of the myocardial tissue, resulting in one of several "mechanical complications" of M.I., which are discussed below. In addition, inflammation surrounding the injured myocardial tissue can lead to post-infarction pericarditis (i.e. inflammation of the lining around the heart).
To better understand the possible complications following M.I., we need to begin with a review of the anatomy of the coronary artery blood supply to the heart. For a review of the coronary blood supply, please refer to the textbook, pages 6 through 8, and refer to the diagram below.

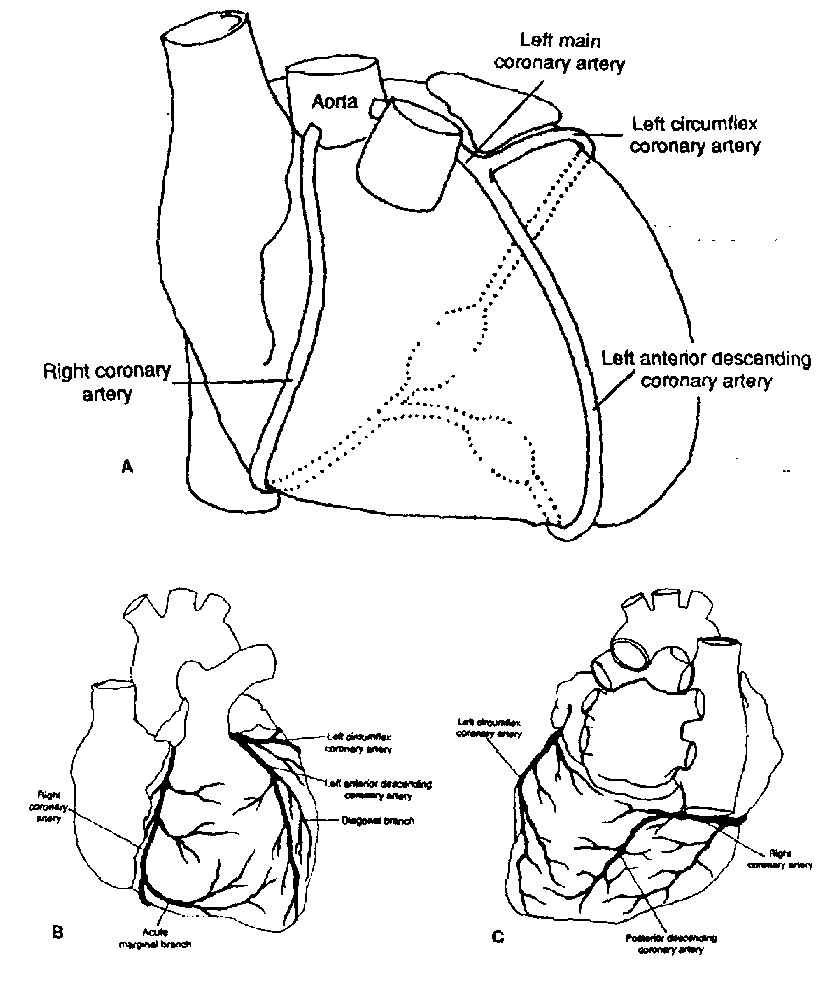
Fig.1-5. Coronary artery anatomy. A, A schematic representation of the right and left coronary arteries demonstrates their orientation to one another; the left main artery bifurcates info the circumflex artery, which perfuses the lateral and posterior aspects of the left ventricle (LV), and the left anterior descending artery, which perfuses the LV anterior wall, the anterior portion of the intraventricular septum, and a portion of the anterior right ventricular (RV) wall. The right coronary artery (RCA) perfuses the right ventricle and variable portions of the posterior left ventricle through its terminal branches. The posterior descending artery most often arises from the RCA. B. Anterior view of the heart demonstrating the coronary arteries and their main branches. C, Posterior view of the heart demonstrating the terminal portions of the right and circumflex coronary arteries and their branches.
With the above anatomical correlates in mind, the various complications of acute MI are easier to explain:
Arrhythmias / Heart block:
Almost any cardiac arrhythmia may occur in the setting of acute MI. Arrhythmias will be discussed more extensively in the EKG lecture, and you can refer to Dr. Lemery's handout.
Briefly, the LAD supplies most of the conduction system below the A-V node (the His-Purkinje system), while the RCA supplies most of the conduction system at and above the A-V node (including the S-A node and the A-V nodes themselves).
Any type of infarct can lead to an abnormal conduction interface (where normal tissue is adjacent to injured tissue), which may lead to re-entry rhythms including ventricular tachycardia and atrial flutter. Likewise, any infarct can lead to impaired LV filling, leading to acute atrial enlargement, leading to atrial arrhythmias including atrial fibrillation.
Hypotension:
Hypotension may occur in various settings following acute MI. These include:
2) Excessive vasodilatation from nitrate therapy
3) Decreased left ventricular filling, secondary to right ventricular infarction
4) Marked reduction in cardiac output due to extensive infarction or to a mechanical complication of MI as described below.
One of the ways that M.I.s can be categorized includes the use of hemodynamic monitoring (Swan-Ganz catheter) to construct Frank-Starling relationships for post-MI patients.
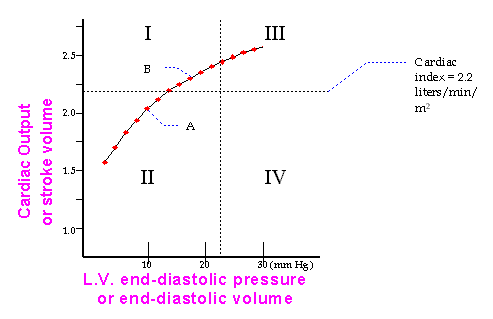
Based on the Starling Curve above, the patientís hemodynamics might be potentially optimized, by managing her fluid status, aiming to keep her in quadrant 1. Inotropes could be added to shift his curve up and to the left. The demarcation lines between the quadrants are drawn at an LVEDP of 20 mm HG, and at a cardiac index of 2.2 liters/min/meter squared.
By definition, patients in quadrant 4 are in "cardiogenic shock" and have a poor prognosis, unless they have a correctable mechanical complication, or unless they are early into the course of their MI, and some of the ischemic myocardium is recovered by early intervention via primary PTCA or emergency CABG.
Mechanical Complications after MI
Some of the potentially correctable mechanical complications of MI are described below, and their peak incidence is usually between 3 and 7 days following MI, when the myocardial tissue is softest, and most vulnerable to rupture.
Acute mitral regurgitation:
Acute MR may occur abruptly from rupture of a left ventricular papillary muscle resulting in a flail mitral leaflet, usually the posterior leaflet. This results in an abrupt decrease in forward cardiac output, leading to congestive heart failure and often to cardiogenic shock.
This occurs more commonly in the setting of inferior MI, since the RCA and circumflex arteries supply the postero-medial head of the papillary muscle, which is more prone to rupture than the antero-lateral head.
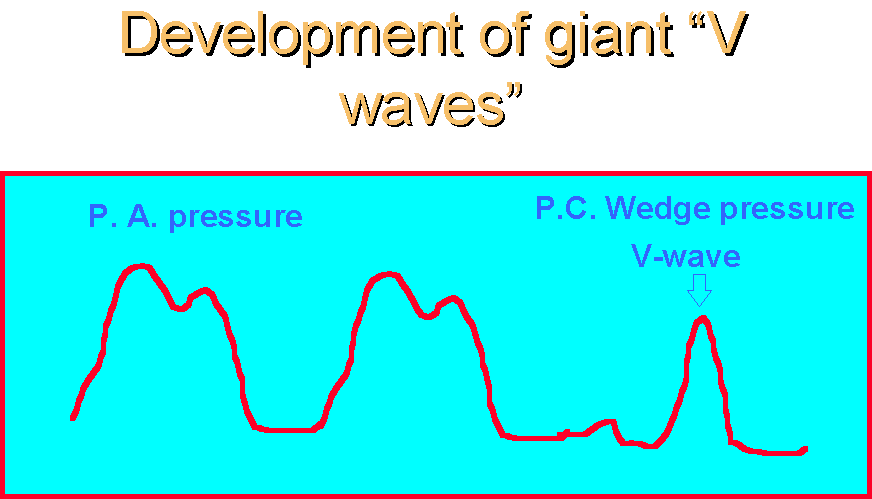
Diagnosis is made by detection of a new systolic murmur, and by the documentation of giant "V-waves" on the pulmonary capillary wedge tracing. Diagnosis may also be made via transthoracic or transesophageal echocardiography, by visualization of the "flail" posterior mitral valve leaflet.
Ventricular septal rupture:
Acute ventricular septal rupture can occur usually several days following the acute infarction, due to softening of the necrotic portion of the septum. This can occur in both inferoposterior and in anterior myocardial infarction. A loud systolic ejection murmur usually occurs and results in an acute left-to-right shunt with congestive heart failure and usually cardiogenic shock.
Diagnosis is made by detection of a new systolic
ejection murmur, often associated with a precordial thrill. Diagnostic
right heart catheterization (Swan-Ganz catheter) will demonstrate an oxygen
saturation "step-up" between the right atrium and the right ventricle of
at least 5%, as exemplified below:
Left ventricular free wall rupture:
Rupture of the left ventricular free wall
is analogous to ventricular septal defect but occurs in the free wall of
the left ventricle, usually resulting in abrupt cardiogenic shock due to
"cardiac tamponade" (see chapter 14, pp 233-236). Rarely a "pseudoaneurysm"
of the left ventricle occurs if there is incomplete rupture of the free
wall and this may be undetected clinically until abrupt deterioration occurs.
Other complications after MI
Left ventricular aneurysm formation:
Left ventricular apical aneurysm formation usually occurs following antero-apical myocardial infarction, after LAD occlusion. This weakening of the apical wall results in an outpouching or "dyskinesis" of the apex of the heart during systole.
The resultant stasis of blood in the dyskinetic segment of the apex may result in thrombus formation and systemic embolization. The reduced left ventricular ejection fraction may lead to congestive heart failure and predispose to ventricular arrhythmias.
Treatment for apical aneurysm formation includes anti-coagulation to prevent embolization, and afterload reduction to reduce LV wall tension.
Right ventricular infarction:
Right ventricular infarction occurs almost exclusively in the setting of right coronary artery occlusion. Hallmarks include elevation of the jugular venous pressure in the absence of pulmonary congestion. Hypotension often occurs in the absence of an elevated pulmonary capillary wedge pressure. Elevation of the ST segments may be present in the right precordial EKG leads.
Treatment includes fluid administration to augment LV filling (since the right ventricle assumes the role of a passive conduit), and to improve LV stroke volume.
Pericarditis:
Post infarction pericarditis usually begins several days after the infarct, due to an inflammatory exudate in the pericardium. Pericarditis pain is distinguishable from infarct pain because of its pleuritic nature, radiation to the left trapezius ridge, and the associated low-grade fever and pericardial friction rub.
Treatment for post-MI pericarditis includes
non-steroidal anti-inflammatory medication, to reduce the pain and inflammation
in the sensitive pericardial tissue.
Cardiogenic shock
Cardiogenic shock results when there is a marked reduction in forward cardiac output leading to hypotension, decreased organ perfusion, and at the same time elevated left ventricular filling pressures leading to congestive heart failure. This can be due to either massive left ventricular infarction or can be the result of one of the mechanical complications described above.
The first step in management is to exclude potentially correctable causes of cardiogenic shock, such as RV infarction (treated with fluid infusions) or mechanical complications which may require surgical repair.
Once these possibilities are excluded, if the patient presents early into the course of MI, acute intervention with PTCA or CABG can be considered, to try to limit infarct size and improve prognosis by emergently re-canalizing or bypassing the infarct-related artery.
If the patient presents late in the course of infarct (i.e. greater than 12 hours after pain onset), management includes supportive measures: i.e. optimizing fluid status via Frank-Starling curves, infusing inotropic agents, and, occasionally, using an intra-aortic balloon pump to mechanically augment diastolic coronary perfusion pressure and reduce afterload.