You will measure the length-tension curve of passive and active gastrocnemius muscle from small rana pipiens, the leopard frog.
Background: The passive
elastic properties of a muscle can be modelled as a nonlinear spring. The force-tension
curve becomes exponentially steeper at longer lengths of stretch. ![]() where σ is stress and ε is strain. Solve for σ !
where σ is stress and ε is strain. Solve for σ !
When muscle is held to a constant length then stimulated by way of it's nerve connection from the ventral spinal cord, the muscle develops additional tension beyond passive. This active force is derived from interactions of the actin and myosin protein filaments. The active force length tension curve is described by a racheting filament model and has its maximum near the muscle's normal rest length. At this length is the most interaction between the actin and myosin filaments, accounting for the largest active force production. At shorter or longer lengths the filaments have less overlap to form cross-bridges; they therefore produce less force.
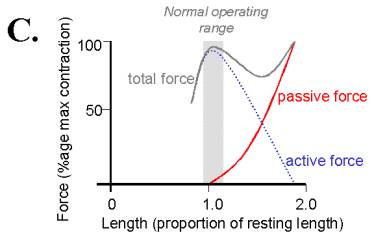
On the diagram above the distance
between the total force and passive force curve is the active force.
Requirements:
(0) Before you go over to B-20 Arnold Lab create a VI that will trigger data
acquisition for a second after the rising edge of a sync pulse is detected.
The one second worth of data will be inspected for MIN and MAX, and displayed
as a waveform on the front panel. If the max frequency component expected is
400Hz, what sample rate should you use?
Not shown is the prior frame for single-point read trigger of sync(trigger
from model 2100 stimulator on rack).
The MIN will be the passive tension, and MAX will be active+passive tension, for a fixed length of muscle (isometric). You should be able to calibrate your VI with a waveform and sync signal from a rm 095 Aglilent waveform generator. [optional: If you're ambitious, add a LP Butterworth filter after the Analog-In icon, with a cutoff set to 20Hz, to attenuate 60Hz hum. Beware: such a filter may cause an artifactual MAX when the DAQ starts...]
Use "Save with options" for your VI created in 095, to make it a 7.0 version (not 7.1); 7.0 is the version machines in Arnold B-20 use.
(0Bi) CALIBRATION: In 095 there will be a ring stand with a MLT500 mounted and attached to a 200 gm weight. The MLT500 is connected to a gain-of-1000 AD524 Inst. Amp, and the multimeter is connected to the output of the 524: Note how much the voltage on the changes when the weight is lifted. You will need the information to translate voltage into tension and to calculate the stiffness of passive muscle.
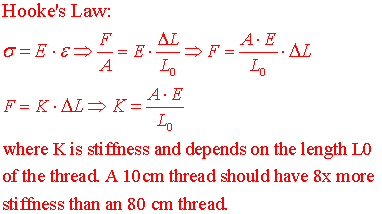
(0Bii) Again, before you leave for B-20, note the approx 75 cm length of green surgical thread (that will be in series with the gastrocnemius muscle) attached to a 1Kg + 100 gm tray. Lift the 1100 gm weight with the thread and measure the ΔL increase in thread length. What will be the stiffness of a 10 cm length of thread that attaches from the tendon to the MLT500 in B-20? See formulas below.
We may set up either the gastrocnemius or sartorius muscle.
(1) Dissection for gastrocnemius
muscle and sciatic nerve:
We'll use rana pipiens, the leopard frog. You are welcome to help with the
dissection (esp if you are premed), or we can do all physiological preparations
for your team:
Pith CNS, and pith down the spinal cord to eliminate unwanted spinal reflexes. Before pithing test withdrawal reflexes of frog for before-and-after comparison. Write down the time of pithing. The frog may give useful responses for up to 2 hours after pithing. Pithing: Insert a 10 cm rigid probe (tapered to a sharp point) through the skin into the foramen magnum between the base of the skull and the top of the vertebrae. (midline, at the level of the caudal tympanic ovals. Pull out and reverse direction of probe to pith spinal cord.
link
to dissection of gastrocnemius
¶Remove skin from leg: Sous chef technique
¶Blunt dissection of "calf" muscle: sew 2-0 silk through beginning
of distal tendon. Tie square knot at the tendon. remaining 2-0 (about 10 cm each
length) to be tied in a loop for attachment to force transducer. Cut tendon away
from bone at a more distal site.
¶Blunt dissection to reveal sciatic nerve from abdominal view. Note sex
of frog (presence of egg sack = female...) Try not to nick open the thoracic
cavity, so the lungs don't collapse. Be mechanically gentle with sciatic nerve.
Pull 2-0 silk thread under the nerve to help lift over stimulating electrodes.
Use Ringer's solution for
exposed tissue. Also known as Ringer’s irrigation: specifically
for amphibians, in one liter distilled water mix:
6.5 g NaCl
0.42 g KCl
0.25 g CaCl2
(2) Force transducer: We will use the ADInstruments MLT500 force transducer,
which can handle up to 500 gm wt force. See
http://www.adinstruments.com/
for information. The pdf link below tells you what color wire goes with which function
on the MLT500. http://www.adinstruments.com/products/datasheets/MLT050.pdf
We will excite the MLT500 bridge from a fixed-voltage triple-output supply. Notice that the data sheets in the pdf file say that 10v is the MAXIMUM excitation voltage to be applied for the MLT500! Use the 5v supply for the excitation voltage!
Because both sides of the MLT500 bridge float w.r.t. ground, we want to make a differential measurement sending each side into a nearby 524 instrumentation amp (gain of 1000). That arrangement will be your "preamp" and allow you to go directly into a single-ended scope input AND a LabVIEW Ain channel (BNC connections). No need for rack preamp or output from back of 'scope...
(3) (perhaps after the data collection...) calibrate the force transducer by hanging a 200 gm weight from it and writing down the resulting DC voltage. It will be in the mV range! (Consider the Tektronix scope as your amplifier, as you did in Lab 8.) What is the unloaded voltage from the MLT500? We will install one our Agilent DMM's in the setup to read off passive voltage continuously, and to make sure the 524 does not saturate.
Mechanics: The MLT500 uses a Wheatstone bridge of 4 strain gauges, arranged on a cantilivered beam of low stiffness. Note where TOP is on the MLT500; One way to measure muscle tension is to suspend the muscle vertically. Another way: use a pulley to redirect horizontal stretch vertical.
The dissection tray must be firmly clamped so it can withstand the upward or sideways force of the stretching muscle.
The force transducer must be held with a micromanipulator, so you can move in millimeter increments to stretch the attached muscle.
Display the force transducer output on the 5110 Tektronix oscilloscope. Trigger the scope from the stimulator. Use DC for recording.
Thread from muscle to force transducer: We don't want to measure the stiffness (or lack thereof) of the thread attached to the muscle. We will use Ethilon #2 braided green surgical thread, which has a good combination of strength, flexibility, friction, and stiffness. We'll tie slip knots with the thread to attach to the transducer.
(4) AM Systems model 2100 stimulator: Examine the controls on the front panel. Begin with a 3 msec duration pulse of 2 mA amplitude, flick output control momentary rocker switch to Manual when a shock is required. Send sync from trigger BNC to EXT on 5110 scope, and to a second LabVIEW Ain channel.
(5) Mechanical arrangement of gastrocnemius and force transducer: Drive two dissecting pins into the base of the dissecting tray, on either side of the proximal tendon, near the frog "knee". Do not push the pins through tissue: the pins will be needed to restrain the frog leg from moving during stretch. Attach the thread loop tied to the distal tendon to the force transducer using a slip knot. Arrange the base of the force transducer micromanipulator such that there is at least 20 cm of pull it can exert from the starting position.
Be sure to measure and record the rest length of your muscle. The length
should be in the range 20-40 mm.
(6) Nerve stimulator: The stimulating electrodes are attached to a Narishige micromanipulator on a stand behind the frog; adjust the electrode pair so the sciatic nerve can be elevated over both electrodes. Make sure no extraneous tissue shorts out the stimulation (clear view underneath the raised nerve). Use single shocks to watch the leg twitch. If you don't see a strong twitch when you stimulate, check the settings on the stimulator, and check that the sciatic nerve is lifted up and electrically isolated on the stimulating electrodes.
To begin with, we won't care to isolate only the fiber headed for the muscle in quesion--we'll watch the whole leg twitch.
Notice where the stimulator "trigger" and the 5110 'scope are connected on the NI rack box; make appropriate matches in your VI for recording the twitch.
(7) Observing twitches on the scope. What is the latency in msec from stimulation to contraction start? contraction max? What is the duration of a twitch?
Slowly increase the stim. from 3msec duration, 0.1mA current, until you observe
maximum twitch tension, then increase the current by another 10% to ensure a
supra-maximal stimulus.
(8) Using your LabVIEW VI, collect data on length vs passive tension and max active twitch tension for the muscle. One lab partner will move the force transducer micromanipulator and flick the stimulator switch. The other partner will call out "ready, go" when the VI has started its search for a trigger, and will record max and min responses on an EXCEL spreadsheet. The MAX raw data you record will be "passive + active" tension.
To insure response over the full range, offset the scope to a negative voltage, so the scope itself doesn't saturate its output. Start with a relaxed setting in which you see no twitch. Don't change the 'scope gain once you start recording data. Stretch by 1 mm at a time and keep repeating the stimulation. You should get to a state of stretch in which the size of the twitch decreases from maximum. You may have to stop at a point where the force transducer has reached its maximum limit.
How repeatable is the data?
Can you notice fatigue or creep of the muscle?
Plot min, max, and max-min (active tension) in EXCEL vs length. Make sure you have
length going "the right direction" from short to long. Arrange that the
length where the twitch is first nonzero is the rest length of the muscle.
Is there any part of the curve with a negative slope? Subtract passive from total
to see the active-only LT curve. Plot the curves in EXCEL, Tension vs Length.
(9) Once you finish verifying your LT curve, cut the sciatic nerve above the electrodes and retest the passive data. Was there any influence on the passive data of a "stretch reflex" mediated by the spinal cord? Or evidence of plastic deformation?
(10) If you have time, repeat the experiment on the muscle of the other leg.
(11) Calculate the maximum passive stiffness of the frog muscle and compare it to your calculation of surgical thread stiffness.
EXTRA: If there is time, insert the pin electrodes from the Blaberus Lab into the gastrocnemius muscle and observe the EMG waveform that results from a twitch-causing stimulation.
Possible FTQ's:
1. In the equation ![]() what do you estimate alpha to be for your passive muscle data?
what do you estimate alpha to be for your passive muscle data?
2. Can you reassemble the 524 preamp circuit if some wires are removed?
3. Explain how sliding filament theory accounts for negative L-T curve.
3. Explain how sliding filament theory accounts for L-T curve below L0.
Reading: pages
8-11 from McMahon, Muscles, Reflexes and Locomotion.
figure from McMahon.
Links: frog legs are a delicacy of French (and Chinese) cuisine. see link. Most frog legs these days come from frog farms in Thailand and Indonesia.
What is the longest muscle in the human body?
To B-20:
DMM
power supply with force transducer & 524 set to gain 1000
surgical instruments: pith rod, scissors, clamps, rat's tooth forceps, sharp
tapered forceps
plastic bags
paper towels
Narishige
In 095
vise for large clamps to hold end of 75 cm surgical thread tied to 1.1Kg weighton
jack stand
power supply with 524 attached to force transducer attached to 200gm weight
on jack stand