Electrodes: Recording, Stimulation.
Metal - Aqueous interface: How current flows through electrolytic fluids (11/08
update)
Topics:
¶ metal-electrolyte reactions
¶ electrode circuits for neural stimulation
Reading:
Any electrode touching tissue for recording should minimize its influence on
the tissue. We want non-reactive metals, like gold, silver or platinum. What
electrochemical property do the nobel metals have that distinguish them?
For recording AC extracellular potentials from single nerve cells a small-size
electrode--in the 10 micron range--is important. Most of the rest of the electrode
may be insulated but the small tip is bare metal. The electrode must also be
etched to a sharp point, to pass smoothly through brain tissue. Thus the need
for tungsten microelectrodes.
*Levick electrodes
*Hubel electrodes
Melted and pulled Pyrex glass tubing to fabricate intracellular microelectrodes:
DC recording
Electrode surface proportional to electrode impedance, and inversely proportional
to spatial localization.
------------------------------------------------------------------------------
Chemical reactions at the metal-aqueous interface
¶electrodes as another kind of "sensor"
¶the nature of skin as a aqueous interface.
Reading: see Derenzo, page 177, and Daniel Harris, Quantitative Chemical
Analysis, W.H. Freeman (1987) chpt 15.
DEMO: a lemon or potato "battery" with various
pairs of metal electrodes: (Fe, Al, Au, Cu, Z, Ag, W...) Zinc, and silver for
example, inserted through the rind results in a voltage of about 1.1v. Imagine
the 1.1v in series with an output resistance.
Place a 20K ohm load between the electrodes on the battery and watch voltage
drop, to reveal the size of the output resistance. What do you calculate the
output resistance to be? How much power can the battery deliver?!
You can work out what to expect from various metal combinations
in aqueous solutions with a Table
of Standard Reduction Potentials
Metal electrodes in water. Imagine one terminal
of a 9v. battery connected to a silver wire in cup of water, and the other terminal
to another Ag wire in the same cup. At the negative terminal two electrons will
combine with 2 H+ ions which will combine to form H2.
At the positive terminal an Ag atom will oxidize, giving up an electron e- and
going into solution as Ag+, basically replacing the lost H+ at the negative
electrode. Will the Ag+ combine with OH- to form AgOH?
The "resistance"
of the water between the electrodes will be relatively high because the concentration
of ions in distilled water is only 10^-7.
Electrode in saline: What will happen
if NaCl is dissolved in the water (creating saline), with 2 battery terminals
in place? see below, where we have added an agar bridge between the electrodes.
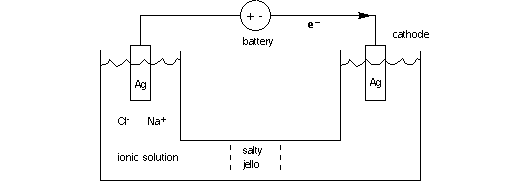
Terminology: If an electrode delivers electrons to a solution it is acting
as a cathode, and oxidation occurs at the electrode. If it accepts electrons
(or negative ions), it is an anode.
Let's follow an electron around the wire to the cathode. Imagine the electron
comes from a reaction at the tip of the silver anode, Ag > Ag+
+ e-. The electron combines with a sodium
ion, removing a positive charge from the solution and creating (briefly) elemental
sodium. The elemental sodium reacts quickly with water to produce sodium hydroxide
and hydrogen gas. You see gas bubbling at the cathode. The sodium hydroxide
ionizes completely.
Na+ + e- --> Na
2 Na + 2 H2O --> 2 NaOH + H2
(gas)
NaOH --> Na+ + OH-
The overall reaction, powered by the battery, can be described as:
2 Ag + 2 Na+ + 2 H2O --> 2Ag+
+2 H2 + 2 Na+ +2 OH-
where there are now two silver ions in solution and 2 more hydroxy negative
ions created, in solution, and H2 gas bubbles; the hydroxy ions represent the
continuation of the free electron current from the wire in the solution. (B&V
vol 3, p. 79)
At the Ag-saline anode electrode the a Cl- ion reacts with Ag to release an
electron: Ag + Cl- --> AgCl + e- . Silver chloride is a stable salt,
and will form a grey coating on the shiny silver wire.
Notice the difference in electrode reactions between distilled water and saline.
In particular, if you try the experiment with a 9v battery and iron nails for
electrodes, you will see a healthy wellspring of bubbles in the case of saline,
but no bubbles in the case of distilled water. Because of the 5 orders of magnitude
difference between ion concentration in H2O and saline, the production of H2
in water is 5 orders of magnitude less. The lack of electrical conductivity
of water leads to its application as a cleaning agent for electrical equipment,
in particular, cleaning insulators near high voltage power lines, with the water
under pressure coming out of fire hoses.
Electroplating: If the solution were silver chloride and the
electrode was, say, tin, then Ag+ would react with the free electron at the
cathode, and would form elemental silver that would plate onto the electrode.
The electrode would become silver plated tin. Thus the cathodic reaction can
in some cases be described as a plating electrode, an important process in the
jewelry industry of Providence.
What happens to the tin (Sn) atom that gave up the electron? It goes into solution
and is replaced by the silver atom. See below for another example:
Reading: Philip
H. Rieger, late of the Chem Dept at Brown, wrote Electrochemistry, 2nd Edition,
Chapman-Hall (1993), DD553.R3. On p. 398 he says, "...to plate
copper on a steel substrate the object to be plated is made the cathode in an
electrolysis cell where a piece of copper is used as the anode. There are many
subtleties, however, which must be considered in practice. Thus, in plating
copper on steel, for example, we immediately recognize that the reaction
Cu2+ (aq) + Fe(s) --> Cu(s) + Fe2+(aq)
is spontaneous. To prevent
the dissolution of iron from the substrate, the activity of Cu2+(aq) must be
reduced by the addition of a complexing agent which coordinates strongly to
Cu(II) but much less so than Fe(II)... In electroplating applications, it is
usually desirable to deposit a layer of uniform thickness. This requirement
is not difficult to meet if the substrate has a simple geometry. If there are
holes or recesses, however, a uniform layer can be quite difficult to achieve."
Rieger goes on to analyze
methods for improving the plating of holes, noting that the addition of inert
electrolyte can lower the IR drop for the current path trying to reach the holes,
and addition of a wetting agent to facilitate the release of H2 bubbles from
the holes.
Etching: If the electrolyte were NaOH (sodium hyroxide), and the anode a
thin tungsten (W) wire, and the other electrode a "carbon reference" (like
the center pole in a D battery) then the anode reaction would etch W atoms
off into the solution.
Demo: The output of a variac connected to tungsten
and reference electrodes, and set at about 10v RMS, can etch the tungsten wire (125
μ diam) to a sharp smooth point, so fine it can't be resolved under a light microscope
(< 1 μ ). Both phases of the AC cycle can etch:
W > W+ e- > and e- + Na+ > Na (Na
combines with H20 then, and generates H2 gas...)
W + Cl- > WCl + e-
such etched electrodes, dipped in epoxy to limit exposed metal to a 20 micron
length, are useful for single unit recording from CNS neurons. At 500 Hz such electrodes
will have about 500K ohms of impedance.
Other versions of such etching reactions can be thought of as corrosion,
and are responsible for the degradation of buried metal pipes in wet soil.
AgCl electrodes: Electrochemical reactions with pure silver are electrically
noisier than the Ag-AgCl reaction. The AgCl coating is a good buffer. The elemental
silver at the surface changes to a AgCl film, which may ionize partially to
Ag+ and Cl- in solution. With AgCl coating on the cathode the reaction changes
to: (B&V vol 3, p 79)
e- + AgCl > Ag + Cl-
thus elemental Ag is removed from one electrode and silver chloride powder ends
up coating the other electrode.
2006. Instrumentation for ion monitoring: Potentiostat:
A potentiostat can keep track of ion concentration by balancing the voltage
between "working" and reference electrodes while passing current through
a control electrode. For example, the amount of gold in solution for electroplating
can be monitored by a potentiostat. (Technics, Cranston RI). see squinchy
tutorial
Reading: Geddes'
Book (R856/G43/1975) pages 198-215
Practical tips for electrodes attached to humans:
Webster book. page 280:
avoid solder (lead Pb), and dissimilar metal contacts
Use good surgical tape and conductive cream
avoid flexing wires at contact, and make sure there is strain relief.
beware of moisture under insulation
Use AC coupling and an isolation amplifier for patient safety
Can abraid the skin with sandpaper to remove a layer
of epidermis and lower impedance.
Smaller electrode size means better spatial localization. Ground electrode can
be a large plate.

