Biomedical Engineers Partner with Toxicologists and Pathologists to Advance Toxicity Testing
The 1st Annual Brown University Bioengineering Research Partnership (BRP) retreat held on April 11th brought together the interdisciplinary research team from Brown’s Center to Advance Predictive Biology (CAPB) with world-leading experts in environmental toxicity testing. The day focused on the first year of progress on the 5-year, $2.5 million grant: Human 3D Microtissues for Toxicity Testing via Integrated Imaging, Molecular and Functional Analyses (U01 ES028184) awarded by the National Institutes of Health (NIH) to PI Kim Boekelheide, Professor of Medical Science and Associate Director of the Superfund Research Program, and the team of bioengineers, biologists, toxicologists, electrophysiologists, and pathologists. Key team members include the Center for Biomedical Engineering’s Jeffrey Morgan, Diane Hoffman-Kim, and Kareen Coulombe as well as Pathology’s Agnes Kane, Cardiology’s Ulrike Mende and Bum-Rak Choi, Bioimaging’s Robbert Creton, and Diagnostic Imaging’s Derek Merck.
The event was opened by Jill Pipher, Vice President for Research, and Larry Larson, Dean of Engineering. Both stressed Brown’s support for the important work of the BRP team. Boekelheide then introduced the External Advisory Committee: Mel Andersen, Distinguished Research Fellow at ScitoVation; Paul Carmichael, Science Leader at Unilever; Steven Ferguson, Scientist in the Molecular Toxicology and Genomics Group within the Biomolecular Screening Branch (BSB) of National Toxicology Program (NTP); and James McKim, President at IONTOX, LLC; the Scientific Steering Committee: Boekelheide; Morgan, Professor of Medical Science, Professor of Engineering at Brown and founder of Microtissues, Inc.; David Savitz, Professor of Epidemiology at Brown’s School of Public Health, Professor of Pediatrics, Professor of Obstetrics and Gynecology at Brown’s Alpert Medical School; and Les Reinlib, health scientist administrator in the Susceptibility and Population Health Branch and director for the Breast Cancer and the Environment Research Program and the Environmental Health Sciences Core Centers at the National Institute of Environmental and Health Sciences (NIEHS); and commercial partners: Perkin Elmer, Unilever, ScitoVation, and Microtissues, Inc. The stage was set for the day by introducing the BRP’s plans to evolve the $3 billion safety testing industry through advances in the new field of in vitro pathology. The BRP’s research is inspired by the 2007 National Academy of Sciences Report: Toxicity Testing in the 21st Century and gained momentum in 2012 when Boekelheide and Morgan partnered in adopting Morgan’s 3D Petri Dish® technology. They pulled in other Brown experts in pathology, tissue engineering, imaging, and machine learning, and with the generous support Brown University and of Donna McGraw Weiss ‘89 and Jason Weiss added tools like the Perkin Elmer Opera Phenix™. Boekelheide quoted Thomas Edison with “The value of an idea lies in the using of it.” With the recent award of the BRP grant, the group stands poised to put their ideas into action.
The audience of students and scientists then learned about breakthroughs and visions in in vitro test systems through 14 multidisciplinary presentations. Highlights included Reinlib’s perspective on the vision of the NIEHS in translating bench science into environmental public health and disease prevention; Patrick McMullen’s, Director of Computational Toxicology at ScitoVation, perspective on tiered toxicity testing and Brown BRP’s positioning in the tiers, and Andersen’s perspective based on more than 40 years in the field that “the times they are a-changin” (Bob Dylan) as he shared the exciting emergence of new alternative methodologies (NAMs) in safety testing.
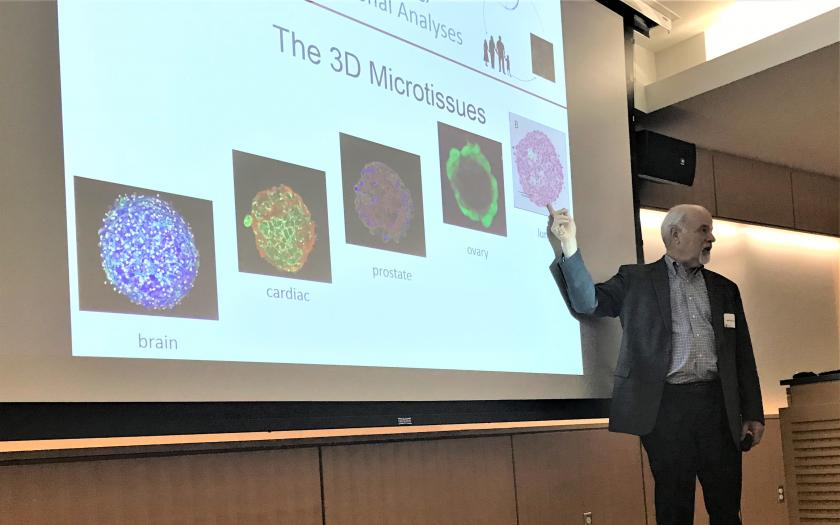
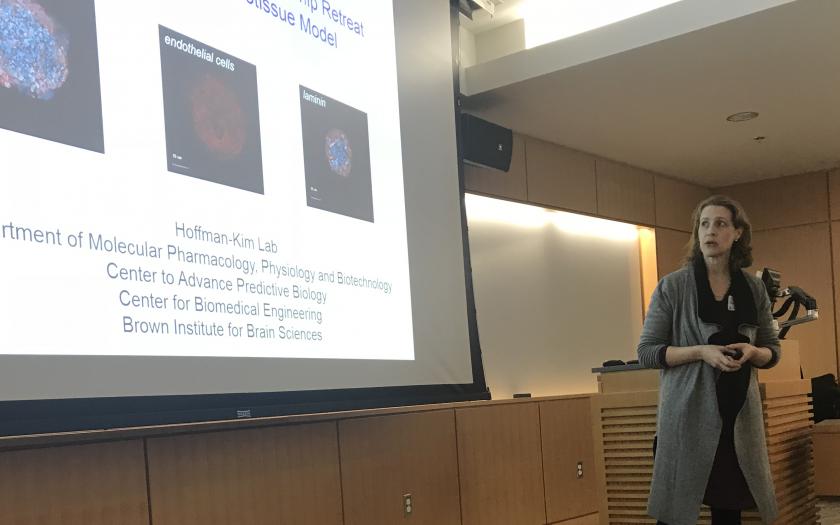
Presentations also emphasized a key feature of the BRP’s research proposal, the development of 3D microtissue models for toxicity testing in 5 different organs: prostate, ovary, lung, brain, and heart. An emerging theme of these presentations was the importance of cell-cell interactions in these models and systems. Development of Boekelheide’s prostate model incorporating epithelial-stromal interactions and performance of functional gap junction assays in Morgan’s ovary model were presented. Kane, Professor of Medical Science, shared her group’s work on incorporating fibroblasts and epithelial cells in their model to elucidate the effects of materials like carbon nanotubes on lung fibrosis. Hoffman-Kim, Associate Professor of Medical Science and Engineering, described efforts in her lab to rebuild some of the key cellular components of the brain: neurons, endothelial cells, and glial cells in a functional model to test for neurotoxicity. The cardiac model is being developed through a collaboration among Coulombe, Assistant Professor of Engineering; Mende, Professor of Medicine; and Choi, Associate Professor of Medicine. Coulombe presented their development of cardiac microtissues including human cardiac myocytes and fibroblasts to screen for causes of life-threatening cardiac arrhythmias.
The BRP’s vision to develop “vision” of 3D models with advanced technologies to generate new insights and standards, building on histopathology as a gold standard for biomarkers, was the focus of the afternoon presentations. Beth Leary (PhD candidate, Biomedical Engineering), Ben Knorlein (Application Scientist, Brown’s Center for Computation and Visualization), and Haitham Seada (Postdoctoral Fellow, Boekelheide Lab) described their efforts to develop the pipeline to generate informative data from numerous large stacks of image files. Kevin Quick, Sales Director (Imaging) at PerkinElmer, described their vision and efforts in 3D high content screening, and Merck, Assistant Professor of Diagnostic Imaging, Engineering, and Radiation Oncology, likened the problem of finding a sick cell in an image to finding a picture of a cat on the internet, a problem that can be solved by applying strategic machine learning.
A recurring theme of the day was the progressive nature of the BRP’s research. Morgan aptly pointed out, “We’re not doing biology on plastic anymore.” During his presentation, McMullen posed the question, “How do we use pieces of information that we can measure to make inferences about adverse effects in humans?” In its first year, the BRP at Brown has made important strides toward developing the types of models and tools needed to gather and connect in vitro pathology with potential impact in safety testing for humans.