Lori Daiello spent the first decade of her career working as a clinical consultant pharmacist in assisted living facilities and nursing homes. She says observing the devastating effects of late-stage Alzheimer’s disease on patients and the prolonged suffering of their families and caregivers was the most impactful experience of her professional life.
“It remains an enduring motivator for my work,” said Daiello, a senior research scientist affiliated with Brown University’s Center for Alzheimer’s Disease Research and the Alzheimer’s Disease and Memory Disorders Center at Rhode Island Hospital.
Daiello, who is also an associate professor of neurology (research) and of health services, policy and practice at Brown, investigates medication effects on memory and functioning, surgery and cognitive impairment in older adults, and the treatment of behavioral symptoms in nursing home residents with dementia. She is leading a landmark study, funded by the National Institutes of Health, designed to understand why changes in memory and thinking can happen following major surgery, and why they are more likely to occur in people 70 years old and older.
The CREATES study follows a cohort of 200 older adults undergoing major elective surgery at Rhode Island and Miriam Hospitals. Daiello’s team is looking at whether people who experience memory changes after major surgeries are at greater risk of developing long-term problems with brain function, such as Alzheimer’s disease.
In recognition of Alzheimer’s Awareness Month, Daiello discussed how the CREATES study is evaluating blood-brain barrier permeability before and after surgery, as well as why the search for new treatments for Alzheimer's disease has been so difficult and where she sees the field going, particularly the development and approval of new drugs and the use of biomarkers to inform treatments and therapies.
Q: What is unique about the CREATES study?
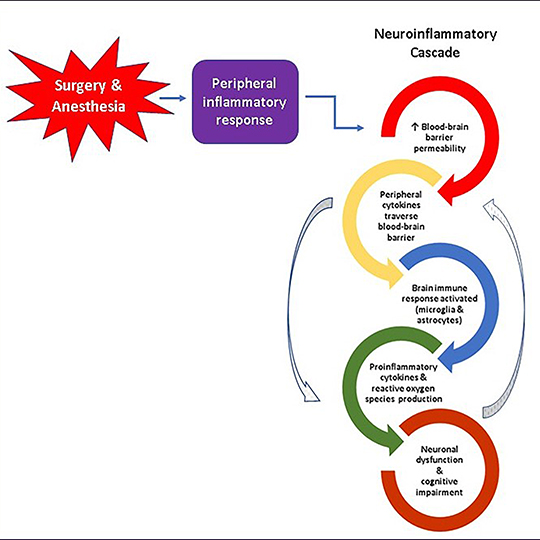
Recent research suggests that the breakdown of the blood-brain barrier, a network of microscopic blood vessels formed by specialized cells that function as the brain’s primary protector and gatekeeper, plays a crucial role in the development of Alzheimer’s disease, and it may also be a risk factor for cognitive dysfunction after surgery. Damage to this barrier can trigger release of proteins that stimulate neuroinflammation and neurodegeneration. Researchers have long recognized the importance of the blood-brain barrier, but non-invasive and accurate methods to assess its health and function have been lacking until recently.
The CREATES study uses a new type of magnetic resonance imaging (MRI) of the brain that measures the passage of water molecules across the blood-brain barrier during a 20-minute scan. This data is used to calculate a mathematical snapshot of blood-brain barrier function. We will use this information, in conjunction with patterns of neuroinflammatory proteins in blood, and results of cognitive tests to investigate long-term changes in cognitive function, and the incidence of Alzheimer’s disease among 200 older adults preparing to undergo major elective surgery at Rhode Island or Miriam Hospitals.
I’ve been surprised and excited by the enthusiasm of our study participants for this research. Many of our volunteers cite a personal connection to someone with Alzheimer’s disease as their primary motivation for enrolling.
Q: Why has the search for new treatments for Alzheimer's disease been so difficult given that so many resources have been invested in this area?
This type of question comes up frequently in my work, and it is asked by people living with Alzheimer’s disease, their families and friends, and by colleagues practicing in other medical specialties. I’ve yet to figure out a brief and uncomplicated answer, but I’ll share a few of my thoughts. A long history of inadequate federal funding for cognitive aging and Alzheimer’s disease research is a major contributor to this problem. Thanks to the tireless advocacy of the Alzheimer’s Association and other organizations, funding from the National Institutes of Health (NIH) for Alzheimer’s disease and other dementias received a notable boost in recent years, with $3.1 billion in 2021. But funding continues to lag the annual expenditures for cancer, heart disease and COVID-19 research.
Aside from money, I think that there are other challenging barriers to making the type of research progress that translates into new and effective treatments. We still lack fundamental understanding of the mechanisms that initiate Alzheimer’s disease neurodegeneration and drive its progression. It’s often said that Alzheimer’s disease is an exceedingly complex disease and effective treatment will likely require multi-drug combinations; however, current clinical trials are designed to evaluate investigational drug monotherapy.
Another avenue to developing effective new treatments may come through advances from ongoing research on defining clinicopathological heterogeneity in Alzheimer’s disease. Reports from post-mortem studies suggest that pure Alzheimer’s disease neuropathology is present in about 30-50% of cases assigned a clinical diagnosis of probable Alzheimer’s disease, while the remainder have varying degrees of other neuropathologies, such as cerebrovascular disease and Lewy bodies. Targeting treatments to specific Alzheimer’s disease subpopulations holds potential for improving outcomes in clinical trials of investigational treatments.
For general context, some may be interested in the 1987 congressional report, Losing a Million Minds: Confronting the Tragedy of Alzheimer's Disease and Other Dementias. It’s a fascinating read and helpful for understanding the magnitude of the advances that have occurred in Alzheimer’s disease research, NIH funding and healthcare policy over the past 35 years. Looking back, I think there is reason for optimism in the coming years, given the focus on accelerating the pace of research progress through diverse initiatives, such as infrastructure support for cross-disciplinary research collaborations, and platforms like the Global Alzheimer’s Association Interactive Network that facilitate data-sharing among scientists.
Q: Where do you see the field of Alzheimer’s disease research going, particularly the development and approval of new drugs and the use of biomarkers to inform treatments and therapies?
I’ve been around long enough to witness major paradigm shifts in Alzheimer’s disease research and drug development—from an emphasis on symptomatic treatments for the dementia phase of the illness, to a primary focus on interventions to prevent or delay the onset neurodegeneration during the pre-symptomatic phase, which precedes the onset of dementia by up to 20 years, using the available biomarkers and molecular imaging. I think the future may bring about another shift, with the impetus on developing new biomarkers that identify an at-risk population earlier in the lifespan—20- and 30-year-old individuals—accompanied by new drugs and lifestyle interventions to prevent Alzheimer’s disease.
