ARCHIVED OSP ANNOUNCEMENTS
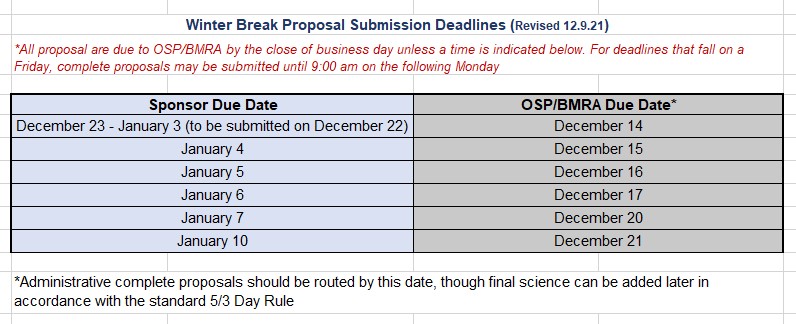
Winter Break Proposal Submission Deadlines (11/1/2021)
Updated 12/9/2021 - In light of the fact that the 2021 Winter Break has been extended by 1 day, our Winter Break Proposal Submission Deadlines have been adjusted. Please see the revised table below:
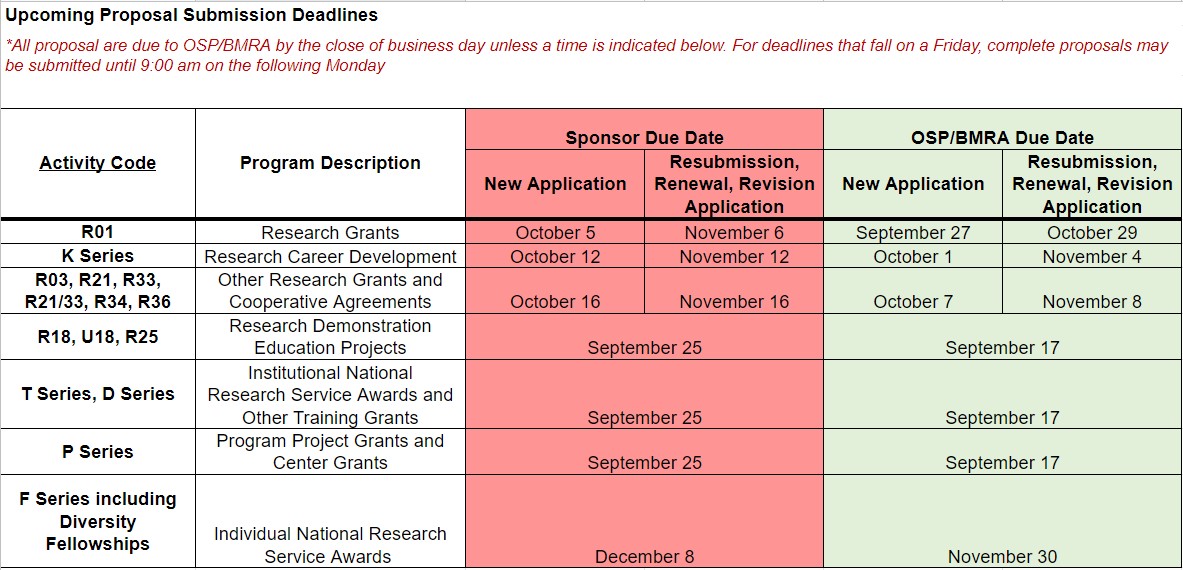
Upcoming Proposal Submission Deadlines for OSP/BMRA Review (8/19/21)
New 3 Day Review Period for Just In Time (JIT) (6/7/21)
Due to the revised NIH guidelines and templates for Other Support, we understand that additional time and effort is required for the completion of Just In Time (JIT) submissions to NIH. In order to ensure that each submission receives an adequate review, OSP requests that JIT submissions which are routed for review after July 1, 2021 are sent at least three (3) full days before the sponsor’s deadline. Please note that similar to our proposal review procedure, this 3 Day Review Period will not be required in cases where the sponsor requests documents with a short turnaround time. Our hope is that setting clear deadlines and expectations will help Investigators, Department Administrators, and OSP staff adequately prepare for the submission of these new documents.
If you have any questions about this requirement, please feel free to email your Pre-Award Contact or [email protected]
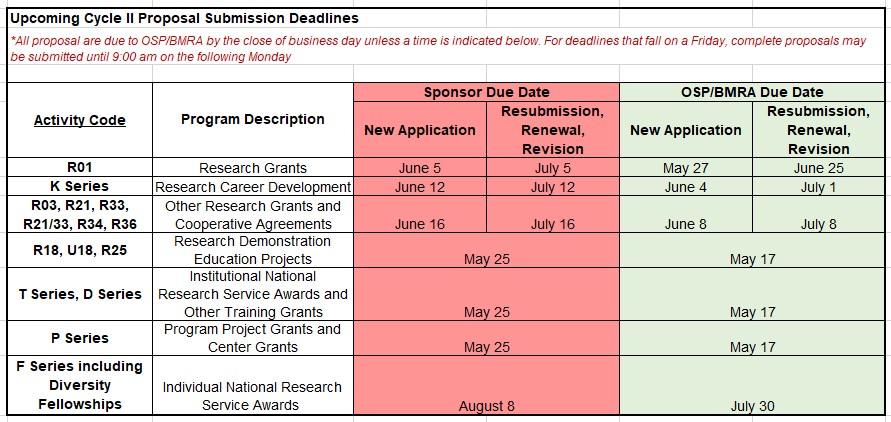
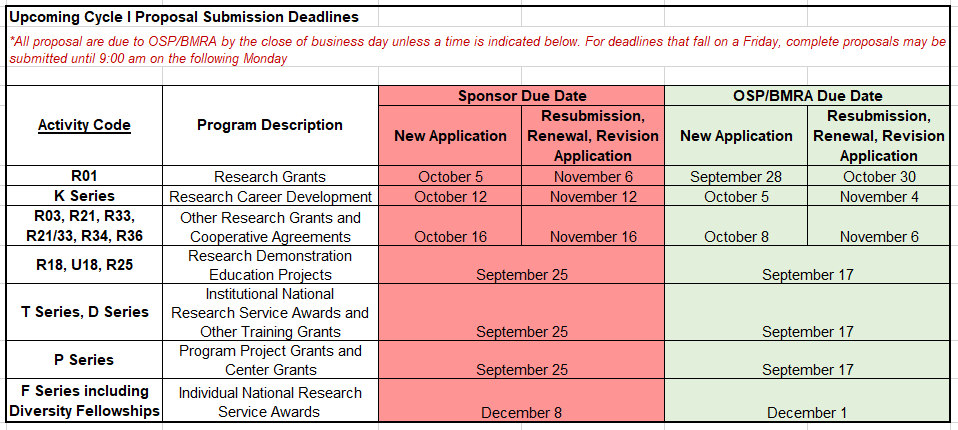
Upcoming Proposal Submission Deadlines for OSP/BMRA Review (4/21/21)
Upcoming Changes to the NIH Biographical Sketch and Other Support Format Page for Due Dates on or after May 25, 2021 (3/16/21)
NIH has updated its application forms and instructions to support the need for applicants and recipients to provide full transparency and disclosure of all research activities, foreign and domestic. Details and FAQs are found at Notice NOT-OD-21-073.html. We have also created a Guidance and Resources page to help explain these changes. This new site contains instructions, samples, and a recording of our recent virtual training.
2021 Salary Cap and Stipend Levels Announced by NIH (2/17/21)
Effective January 3, 2021, the NIH & AHRQ Salary Cap is set at $199,300, please see NIH Guide Notice NOT-OD-21-057
Ruth L. Kirschstein National Research Service Award (NRSA) Stipends, Tuition/Fees and Other Budgetary Levels have also been adjusted for Fiscal Year 2021. This Notice establishes stipend levels for fiscal year (FY) 2021 Kirschstein-NRSA awards for undergraduate, predoctoral, and postdoctoral trainees and fellows.
Please see the updated Salary Cap Worksheet, which can be found here.
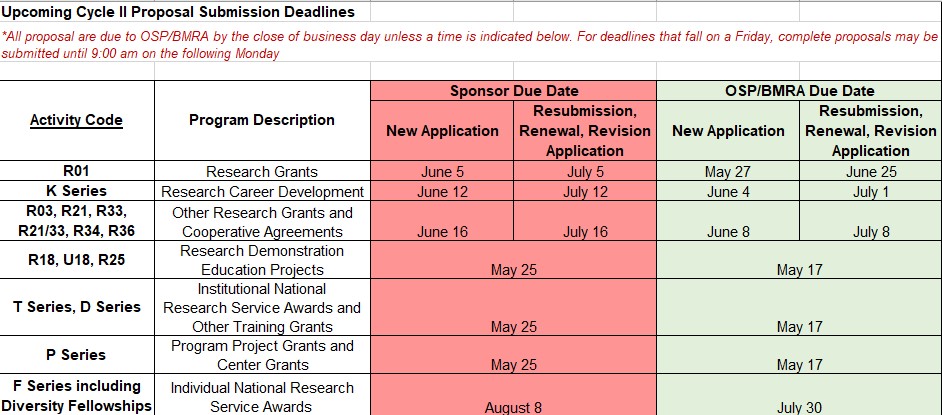
Upcoming Proposal Submission Deadlines for OSP/BMRA Review (1/14/21)
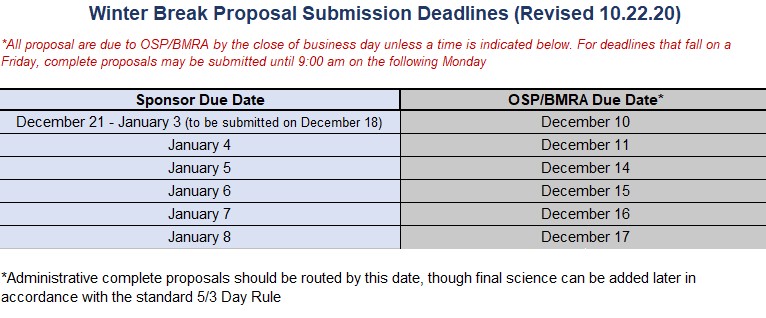
Looking Ahead: Winter Break Proposal Schedule (updated 10/22/20)
NASA Science Mission Directorate - COVID Flexibilities beyond 9/30/20 (10/2/20)
Per NASA Grant Notice 20-02, requests will be considered from award recipient(s) stating that they have experienced a loss of operational capacity due to the COVID-19 pandemic and that they need to charge salaries or benefits to an active NASA award consistent with their policies of paying salaries under unexpected or extraordinary circumstances. Verify this in advance with your organization's authorized organization representative (i.e., OSP Grant & Contract Administrator or BMRA). See this link for more information and web form.
Department of Energy Office of Science - Response to Delayed Progress in Research Caused by COVID-19 (10/2/20)
The Director of DOE's Office of Science (SC) writes "SC is committed to maximum flexibility in administering awards, recognizing potential delays in research caused by impacts of the COVID-19 pandemic. Investigators will not be penalized for such delays. Researchers are encouraged to make the best progress possible in their changed, and changing, situations." Read a full copy of the statement here and visit the Dept of Energy's Responding to COVID web site here.
Upcoming Proposal Submission Deadlines for OSP/BMRA Review (9/15/20)
New Assistant Director, Post-Award Services Named (9/15/20)
We are delighted to announce the promotion of Robin Eubank to the Assistant Director, Post-Award Services position in the Office of Sponsored Projects. Robin brings over 14 years of experience as an Auditor with supervisory responsibilities, Internal Control and Financial Statement analytical skills as well as technical GAAP expertise. In addition, Robin led client engagements in non-profit and educational sectors including performance of Single Audit compliance testing on federal and federal pass-through funding. Since arriving at Brown in 2018, Robin has led the Effort Certification process, prepared the NSF HERD Survey and the NIH Biomedical Research and Development Price Index Report submitted on behalf of the University to Brown's largest external sponsors of research. She has also led several initiatives for the OVPR’s Diversity and Inclusion Committee.
Please join us in congratulating Robin on her new role in the Office of Sponsored Projects.
Management of Fixed Price Agreement Residual Funds (8/11/20)
To manage residual funds remaining on sponsored project awards that are issued to Brown as Fixed-Price Agreements, the Office of Sponsored Projects (OSP) has adopted the following procedures:
At award close-out, OSP will review the account to ensure that all sponsored funds have been received, all expenses related to the project are reflected in the account, all PI and personnel effort that has been charged to the project is in line with the work performed, and all award requirements (e.g., final technical reports) have been met.
OSP will confirm that the terms and conditions of the award do not require the return of unspent funds. OSP will identify direct and indirect costs by applying the project’s established Facilities & Administrative (“F&A”) rate to total unspent funds using the F&A rate in effect during the final year of the project.
The residual funds will be defined as either small or large balances:
-
Small balances: A balance after award closeout <25% of the total sponsored award amount and/or <$100K.
Small balances will be distributed during the award close-out process with direct costs transferred to the PI and the applicable indirect costs transferred per the University’s F&A revenue allocation methodology.
-
Large balances: A balance after award closeout ≥25% of the total sponsored award amount and/or ≥$100K.
Justification of large unspent balances by budget line item will be completed on the Residual Funds Balance Transfer Form along with confirmation that all project deliverables have been met. The Form will be forwarded to the OSP Grant/Contract Accountant.
Once the Balance Transfer Form is accepted by OSP, direct costs funds will be distributed to an account identified by the PI and endorsed by the Dean/ Department Chair. The F&A portion of the large balance will be transferred per the University’s applicable F&A revenue allocation methodology.
If multiple PIs/Departments are named on the Award, there will be a fair distribution among the parties as determined by OSP in consultation with the lead PI.
For questions or concerns, please contact [email protected], Director, OSP, or [email protected], Associate Vice President for Research.
Use of Electronic Signatures on Proposal and Award Documents (8/7/20)
OSP is paper-free! As a reminder electronic signatures were formally adopted by OSP in 2019. OSP uses "e-signing" on outgoing subaward agreements and amendments in addition to standard grants & contract documents. For example, many foundations now require the use of the Docusign tool for award endorsement.
At the proposal stage, we encourage electronic signature on internal forms including Investigator Certifications when a Certified Digital Signature is used. Using Adobe’s “Fill & Sign” tool is not secure or certified, so these signatures cannot not be accepted. Instead, use the Digital Signature option in the Adobe “Certificates” tool. Adobe's instructions can be found here and we've prepared our own instructions here.
If you have questions or a digital signature isn't possible for whatever reason, please reach out to your OSP Representative and we'll find a solution.
New F&A (Indirect Cost) Rate Agreement is Published (7/17/20)
Important update on Brown’s negotiated F&A rate for sponsored projects. Over the next 3 years, the On-Campus Research rate will increase each year in FY22 and FY23:
FY20 62.5% (July 1, 2019 – June 30, 2020)
FY21 58.5% (July 1, 2020 – June 30, 2021)
FY22 59.0% (July 1, 2021 – June 30, 2022)
FY23 59.5% (July 1, 2022 – June 30, 2023)
“Other Sponsored Activities” will increase from 23% in FY20 to 27% in FY21-FY23.
“Off-Campus Activities” will remain the same for FY20-FY23 at 26%.
New awards beginning on or after July 1, 2019 will start at 62.5% through June 30, 2020; starting on July 1, 2020, they will decrease to 58.5% for FY21, increase to 59.0% FY22, and increase to 59.5% starting on July 1, 2022 for FY23 and forward until a new rate agreement is approved).
Note: This means that for awards that are obligated for several years at once, the account(s) may have up to four IDC effective dates and rates, as our rate changes each fiscal year.
What about Non-competing Continuations? Awards with a start date before July 1, 2019 will continue at 62.5% for the remainder of the competitive segment. Therefore, Noncompeting Continuations for awards beginning prior to FY20 (July 1, 2019) will not be affected, and we can continue using the IDC rate of 62.5% during the continuation period.
What if the Notice of Awards (NOA) lists the incorrect IDC rate? OSP will go back to the sponsor to request a correction in the IDC rate only if: (a) the error results in the overall award amount being reduced inappropriately; or (b) our old rate of 62.5% is listed for FY21 and FY22, and the NOA is received more than 30 days after our new rate agreement has been finalized.
NSF Proposal and Award Policy and Procedures Guide (PAPPG) effective June 1, 2020 (6/15/20)
NSF has made the decision to delay the requirement to use NSF-approved formats for the biographical sketch and current and pending support sections of NSF proposals until October 5, 2020. Proposers must continue to format these documents in accordance with PAPPG requirements (see PAPPG sections II.C.2.f and II.C.2.h).
Webinars covering the use of NSF-approved formats as well as all of the significant changes to the PAPPG are available on the NSF Policy Outreach website.
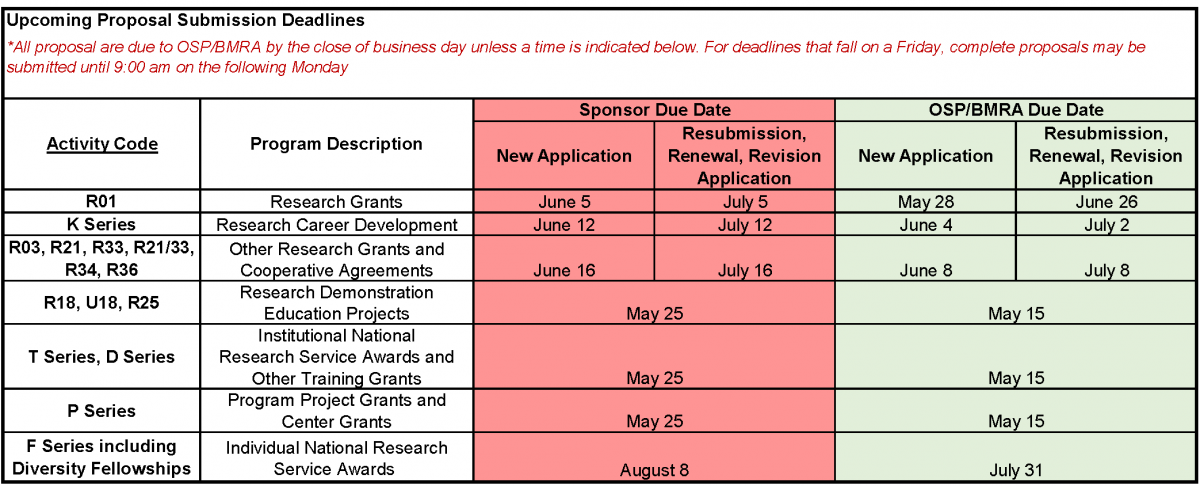
Upcoming Proposal Submission Deadlines for OSP/BMRA Review (5/11/20)
New Associate Director, Post-Award Services Named (5/8/20)
We are very delighted to announce that Heather Dominey has accepted the Associate Director, Post Award Services position in the Office of Sponsored Projects. Heather brings 16 years of Brown financial and accounting experience to the position. She began her career in the Center for Statistical Sciences and moved to OSP in 2009 joining the Post Award Team. Heather has served in positions of increasing responsibility both at Brown and in national professional organizations. She received the Brown University Excellence award for Service in 2014 and the NCURA Region I Merit Award in 2019. Heather holds a B.S. from St Joseph’s College in Maine and an MBA from Johnson & Wales University in Providence.
NIH Salary Cap Increase FY20 (2/6/20)
On February 5, 2020, the NIH issued Notice NOT-OD-20-065, Guidance on Salary Limitation for Grants and Cooperative Agreements FY 2020 announcing that the Executive Level II salary cap increased to $197,300 effective January 5, 2020. The new salary cap applies to awards from the NIH, CDC, AHRQ, SAMHSA, and other DHHS organizations.
NSF Issues FY 2020 Proposal and Award Policies and Procedures Guide (2/4/20)
The Proposal & Award Policies & Procedures Guide (PAPPG) (NSF 20-1) will be effective for proposals submitted or due, and awards made, on or after June 1, 2020. Significant changes include, but are not limited to:
-
Chapter II.C.2.f, Biographical Sketches, has been modified to require use of an NSF-approved format in submission of the biographical sketch. NSF will only accept PDFs that are generated through use of an NSF-approved format. More on the SciENcv
-
Chapter II.C.2.h, Current and Pending Support, has been modified to require use of an NSF-approved format in submission of current and pending support information. NSF will only accept PDFs that are generated through use of an NSF-approved format.
-
Chapter II.E.1 and 2, Rapid Response Research (RAPID) Proposal and EArly-concept Grants for Exploratory Research (EAGER) Proposal, have been supplemented with language which clarifies how RAPID and EAGER proposals may not be used. A new requirement that email documentation from a cognizant NSF Program Officer approving submission of a RAPID or EAGER be uploaded to the proposal also has been added.
2020 Effort Reporting Schedule (12/10/19)
The highly anticipated 2020 effort reporting schedule is now available! Please follow this link to access the schedule and plan ahead. Contact Robin Eubank at [email protected] with any questions.
2020 Principal Investigator Eligibility Policy is released (11/27/19)
A new Principal Investigator Eligibility Policy will go into effect on January 1, 2020. The Policy, PI Approval form and a summary Chart are available here.
OSP/BMRA Holiday Deadlines (11/13/19)
Proposal deadlines following Thanksgiving Holiday. Please be mindful of the University's closure on November 28th and 29th when planning your proposal timelines. Since 5 full days are required for review of proposals, you may need to adjust your proposal routing process to allow for these holiday closures. As an example, proposals due to the sponsor on December 2nd would therefore need to be routed to OSP/BMRA by 5pm on November 20th.
Winter Break Deadlines
Proposals due over the University's Winter Break (between December 21 and January 1st) must be submitted to the sponsoring agency on Friday, December 20th and are therefore due to OSP/BMRA by 5pm on December 12th. This will allow enough time for the review and approval process to be completed.
Due to the short time period between Brown's reopening and the January 7th NIH deadline, we recommend that all submissions for that deadline are routed for an initial review before Winter Break. For these submissions, administratively complete proposals should be routed to OSP/BMRA by 5pm on December 17th. Final science for these proposals will not be due until 5pm on Thursday, January 2nd.
Terminated Workers Effort Certification Business Process Update (11/13/19)
With the new process, the effort reports for terminated employees will still be generated either as the termination notice is received by the Effort Certification Administrator (ECA) or when effort reports are generated through the regular scheduled reporting cycle. The revised business process for the certification of terminated employee effort reports is summarized as follows:
Terminated Worker Effort Certification Routing Process if PI is Active
- ECA will generate the effort report (as currently done today).
- ECA will reassign the effort report to the Effort Certification Partner (ECP) or Cost Center Manager (CCM) in Workday.
- ECP or CCM should review the report, make any necessary changes, and submit the effort report.
- Effort report will route to the PI for certification.
Terminated Worker Effort Certification Routing Process if PI is Inactive
- ECA will generate the effort report (as currently done today).
- ECA will reassign the effort report to the Effort Certification Partner (ECP) or Cost Center Manager (CCM) in Workday.
- ECP or CCM should review the report, make any necessary changes, and submit the effort report.
- Effort report will route to WD Operations once submitted.
- ECA will communicate with department personnel to determine the appropriate person with firsthand knowledge of the worker's effort that can certify the report.
- ECA will work with WD Operations to forward the effort report to the appropriate person for certification.
Upcoming Proposal Submission Deadlines for OSP/BMRA Review (9/16/19)
*UPDATE* please note the updated date for F Series and Diversity Fellowships due to Holiday Closures 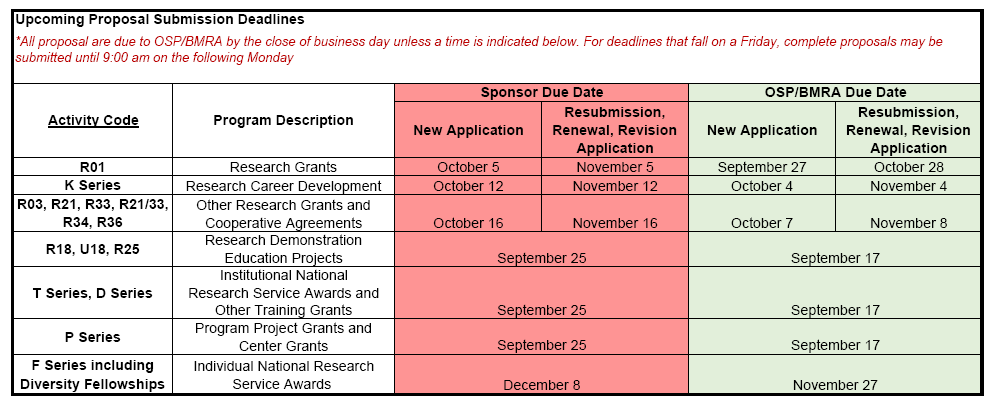
Federal Agency Guidance on Notification and Reporting of Discrimination and Harassment (9/5/19)
Brown University (“Brown” or “University”) is committed to creating and maintaining an educational, research, working, and living environment free from all forms of unlawful harassment and sexual misconduct. The University has created policies and procedures that describe the community standards of conduct as well as the procedures for grievances and complaints alleging violations of its discrimination and harassment policies.
In addition to University policy, Federal and State sponsors of Brown’s research have notification and reporting requirements related to sexual harassment, harassment, sexual assault, and discrimination. Many of these sponsors have recently issued rules and guidance regarding these requirements and the importance of adhering to them.
The specific Federal Agency Guidance can be found here.
Reminders of NIH Policies on Other Support and on Policies related to Financial Conflicts of Interest and Foreign Components (7/10/19)
NIH has released a detailed reminder to the extramural community about the need to report foreign activities through other support, foreign components and financial conflict of interest. NOT-OD-19-114.html contains an expanded clarification and detailed FAQs. At this time, NIH is currently in the process of updating the Other Support Format Page and instructions. The requirements provided in the notice will likely evolve further. Should you have any questions, contact OSP or BMRA Pre-Award Staff.
NSF approves and encourages SciENcv Biographical Sketch Format for Proposal Submissions (6/18/19)
NSF released a communication on Monday stating "National Science Foundation (NSF) has designated the National Institutes of Health’s SciENcv (Science Experts Network Curriculum Vitae) as an NSF-approved format for submission of biographical sketch(es) and is encouraging its use to prepare a biographical sketch for inclusion in proposals to NSF. The following website resources may be of assistance to proposers preparing a biographical sketch using the SciENcv format":
- SciENcv Background
- YouTube Video: SciENcv Tutorial
- YouTube Video: Integrating with ORCID
- SciENcv Help
Important Proposal Submission Update (5/20/2019)
After an 8 week pilot period where we tested the efficiency and effectiveness of submitting proposals in sponsor's systems, OSP and BMRA have determined that proposal submission via sponsor systems does not have a negative impact on either the proposal preparation or the review and submission processes. As such, we are announcing a change to our submission guidelines.
Effective today, proposals that are eligible for System-to-System submission can either be submitted in COEUS or in a sponsor system such as ASSIST, FastLane, or Workspace. There will no longer be a requirement that S2S-eligible proposals be submitted via COEUS.
Please note that regardless of which submission method is used, COEUS remains our official proposal repository. For this reason, a COEUS record will still be required at the time of submission for all proposals.
We've developed an FAQ to accompany this announcement, but please also feel free to reach out to your Pre-Award contact with any questions you may have.
2019 NIH Salary Cap Increase (5/6/2019)
The Executive Level II salary cap used by the Department of Health and Human Services (which includes NIH) has been increased to $192,300. This change is effective retroactive to January 6, 2019, so please be sure to use this new rate in NIH and AHRQ proposals going forward.
More information from NIH can be found here: NOT-OD-19-099.
If you have any questions, please feel free to call or email your OSP/BMRA Pre-Award contact.
Identifying all Sources of Support in Proposals for External Funding (3/14/19)
Federal sponsors are focusing more closely on the full disclosure of project support than in years past. There is concern about ‘under-reporting’ of available research funding and of foreign sources of research support (i.e., foreign components, defined below). It is critically important that complete and accurate information about research support is included in all grant and contract proposals whenever required. This requirement will vary from sponsor to sponsor, so be sure to include other support information in conformance with each Agency’s instructions. Please keep reading for further information here.
_______________________________________________________________
Archived COVID-19 Related Notices
NIH | NSF | DOE | DOD | NASA | NEH | FDA | DHHS | NEA | CDC | AHRQ
back to most recent COVID-19 Related Notices
NIH
NIH Implementation of OMB Memorandum M-20-26 "Extension of Administrative Relief for Recipients and Applicants of Federal Financial Assistance Directly Impacted by the Novel Coronavirus (COVID-19) due to Loss of Operations" (6/29/20)
Applicant/Recipient COVID-19 Update History Please note important updates on May 18, 20, 21, 22 in particular (5/22/20)
NIA Late Application Policy for NIA-Specific FOAs with Application Due Dates in May, June, and July 2020 (5/14/20)
NIH Late Application Policy for Institutional Training Grants to PA-20-142 and PA-20-162 Due to Public Health Emergency for United States Coronavirus Disease 2019 (COVID-19) (5/14/20)
Roundup of New COVID-19 Resources for NIH Applicants and Recipients: Part 2 (4/24/20)
Information for NIH Applicants and Recipients of NIH Funding Related to COVID-19 - updated powerpoint (4/24/20)
Applicant/Recipient COVID-19 Update History (4/2/20)
UPDATE: NIH Late Application Policy Due to Public Health Emergency for United States for 2019 Novel Coronavirus (COVID-19) (3/27/20)
Dr. Mike Lauer, COVID-19 Flexibilities for the Research Communities - Video dated 3/26/20 (3/27/20)
Applicant/Recipient COVID-19 Update History - includes Notices of Special Interest (NOSI) (3/27/20)
NIH's Deputy Director for Extramural Research, Dr. Mike Lauer's blog and video discussing resources for applicants and grantees (3/19/20)
Guidance for NIH-funded Clinical Trials and Human Subjects Studies Affected by COVID-19 (3/17/20)
Coronavirus Disease 2019 (COVID-19): Information for NIH Applicants and Recipients (3/16/20)
NIH Flexibilities Available to Applicants and Recipients of Federal Financial Assistance Affected by COVID-19 and FAQs (03/13/19)
NIH late Application Policy Due to Public Health Emergency for United States for 2019 Novel Coronavirus (COVID-19) (03/10/20)
NIH Guidance on Travel and Meetings Hosted by NIH (3/10/20)
NIH Extramural Response to Natural Disasters and Other Emergencies and FAQs (3/10/20)
Most Recent NIH COVID-19 Related Notices
back to top
NSF
Coronavirus Information
Impact on Existing Deadline Dates
NSF Implementation of OMB Memorandum M-20-26 "Extension of Administrative Relief for Recipients and Applicants of Federal Financial Assistance Directly Impacted by the Novel Coronavirus (COVID-19) due to Loss of Operations" (6/29/20)
NSF PAPPG (effective June 1, 2020) Biographical Sketch and Current and Pending Support Format Implementation Delay (6/15/20)
FAQs regarding the NSF Dear Colleague Letter on the Coronavirus Disease 2019 (COVID-19) (NSF 20-052) (4/7/20)
Revision NSF Implementation of OMB Memorandum M-20-17 (items #8, 10, 12) (4/2/20)
NSF Letter to Community Regarding COVID-19 Important Notice No. 146 (3/23/20)
NSF Implementation of OMB Memorandum M-20-17 (3/23/20)
NSF Detailed Guidance on the Coronavirus (COVID-19) (3/12/20)
NSF Dear Colleague Letter on the Coronavirus Disease 2019 (COVID-19) 20-052 and FAQs (3/9/2020)
Most recent NSF COVID-19 Related Notices
back to top
DOE
PF 2020-20 COVID-19 Guidance for Financial Assistance Actions(4/1/20)
Department of Energy Office of Science - Accommodating Interruptions from Coronavirus Disease 2019 (3/16/20)
DOD
DoD Financial Assistance Recipients Affected by the Loss of Operational Capacity and Increased Costs due to the COVID-19 Crisis (4/13/20)
DARPA COVID-19 Guidance USD (R&E) - Grants & Cooperative Agreements (4/1/20)
Office of Naval Research (ONR) Coronavirus Assistance and Acquisition-Related Information and Resources (4/1/20)
United States Army Medical Research Acquisition Activity (USAMRAA) COVID-19 FAQs (Updated 3/30/20)
Frequently Asked Questions for DoD Research Proposers and Awardees Impacted by the Novel Coronavirus (COVID-19) (3/24/20)
Frequently Asked Question (FAQ) about COVID-19 (Coronavirus) for DARPA Performers (3/23/20)
NASA
Closure of NASA Centers, Laboratories and Test Facilities for NASA Grant or Cooperative Agreement recipients due to Coronavirus (COVID-19) situation (3/30/20)
National Aeronautics and Space Administration HQ - Assistant Administrator for Procurement Message on Coronavirus (3/16/20)
NEH
FAQs – Funding for NEH Applicants and Grantees Impacted by the Coronavirus (3/30/20)
National Endowment for the Humanities - Information on NEH and COVID-19 (3/16/20)
FDA
Food & Drug Administration (FDA) Guidance on Conduct of Clinical Trials of Medical Products during COVID-19 Pandemic (3/19/20)
DHHS
Preparation for Potential COVID-19 Impact on Contract and Contractor Performance (3/23/20)
NEA
National Endowment for the Arts (NEA) FAQs and Information for Applicants and Grantees in response to COVID-19 (Updated 3/31/20)
CDC
Information for CDC Applicants and Recipients of CDC Funding (3/25/20)
Flexibilities Available to Applicants and Recipients of Federal Financial Assistance Affected by COVID-19 (3/25/20)
General Funding and Grants Frequently Asked Questions (3/25/20)
AHRQ
AHRQ (Agency for Healthcare Research and Quality) Flexibilities Available to AHRQ Recipients and Applicants Directly Impacted by the Novel Coronavirus (COVID-19) Due to Loss of Operations (4/10/20)
