New Investigator/Research Staff Onboarding | QA/QI Activities | HRPP/IRB Metrics | Protocol Record Keeping, Best Practice Reviews and Sessions
Quality Assurance / Quality Improvement Program
Brown’s Quality Assurance / Quality Improvement Program (QA/QI Program) is housed within the Office of Research Integrity and reports to the Senior Director of the Office of Research Integrity. The goal of this program is to promote a culture of integrity and excellence at Brown related to human subjects research, and to provide exceptional support and services to our research community in the form of education and outreach.
The QA/QI Program objectives are two-fold:
-
To support institutional regulatory compliance; and
-
To educate and assist the Brown research community in the conduct of compliant research to promote research excellence and reduce administrative burden
Associated with these overarching objectives, the QA/QI Manager will facilitate the conduct of research at Brown by serving as a resource to researchers and the IRB.
New Investigator/Research Staff Onboarding Session
New to Brown Research?
-New faculty?
-New advisor?
-New to Human Subjects Research?
Schedule a "New Investigator/Research Staff Onboarding Session" with our QA/QI Manager, Christiana Provencal. This session provides a one-on-one, in-person (or via phone / Zoom) on-boarding meeting to assist in orienting new investigators and staff to the Brown HRPP/IRB website, forms, processes and policies. Those who have participated in these sessions and responded to a brief survey have offered very positive feedback, with 94% of respondents providing a rating of "very good" or "excellent" regarding the overall helpfulness of the session and 100% of respondents stating they would refer others to the session.
QA/QI Activities
-
Conduct random, on-site reviews to assess regulatory compliance and human subject safety for approved studies and to ensure research teams are maintaining compliance.
-
Provide investigators with quality improvement recommendations to ensure that research is conducted in accordance with best practices.
-
Conduct directed (for-cause) audits at the request of the IRB or authorized Institutional Official. Directed audits focus on areas of concern that have been identified or reported. Recommend actions to the IRB based on on-site observations.
-
Investigate allegations and findings of non-compliance. Report potential serious or continuing non-compliance to the IRB and Institutional Official and propose corrective actions.
-
Provide general guidance or study-specific services at study start-up or throughout the lifetime of a project. These services can include On-site Reviews, Study Consultations, In-services, External Audit Preparation, and Study Management Tools.
-
Assess the performance of HRPP operations via metrics and use these data to design and implement targeted and broad-based quality improvement activities.
-
Assess the performance of the IRB and its membership.
HRPP/IRB Metrics
Median Turnaround Times | Number of Active Protocols
The QA/QI program tracks and publishes metrics as an important element of monitoring IRB and HRPP performance and assessing the impact of quality improvement activities. The initiative to create transparency and to engage the Brown research community in quality assurance and quality improvement activities aimed at increasing efficiency, facilitating compliance, and maintaining excellence in human subjects research, kicked off in earnest starting in fiscal year 2020 and has continued to expand throughout the current fiscal year.
The QA/QI program works collaboratively with the Human Subjects Research Protection Program (HRPP), the Institutional Review Board (IRB), and the research community to ensure support and guidance relative to several aspects of compliance related to federal and state regulations, sponsor terms and conditions, as well as Brown University policies and procedures to ensure compliance with all applicable activities and standards. Evaluation of effectiveness and progress toward achieving stated compliance goals will rely on internal quantitative data, as well as qualitative data gathered from the HRPP, the IRB, and the research community.
Median Turnaround-Times (Submission to Approval) in Calendar Days
Starting in November 2019, the QA/QI program began publishing monthly median turnaround-time (TAT) data, benchmarked against Association for the Accreditation of Human Research Protection Programs, Inc. (AAHRPP) median data for academic institutions. The AAHRPP data represent the Brown HRPP's target turnaround times for each review type. The data noted below includes the actual Brown University monthly TAT for CY 2023. This data will be updated periodically in an effort to provide the research community with information that may be helpful when anticipating the TATs of future submissions. Currently AAHRPP's published data reflect calendar year 2022 , the latest data available. (Click here for Brown University Data for CY 2022.)
|
Median Turnaround Times - New Protocols - Calendar Year 2023 |
AAHRPP Benchmark Data 2022 |
January '23 |
February '23 |
March '23 |
April '23 |
May '23 * * |
June '23 |
July '23 |
|
New Full Board Protocols |
42 |
14 |
24 | N/A | 73 | 23 | 49 | 54 |
|
New Expedited Protocols |
18 |
54.5 |
78 | 21 | 44.5 | 10 | 22 | 22 |
|
New Exempt Protocols |
10 |
13.5 |
16 |
15.5 |
23.5 |
7 |
20 |
28 |
|
Median Turnaround Times - New Protocols - Calendar Year 2023 |
AAHRPP Benchmark Data 2022 |
August '23 |
September '23 |
October '23 |
November '23 |
December '23 |
|
New Full Board Protocols |
42 |
50 | 82 | 62 | 83 | N/A |
|
New Expedited Protocols |
18 |
33 | 36 | 50 | 34 | 36 |
|
New Exempt Protocols |
10 |
27 |
38 |
32 | 35 | 20 |
** Transition to Huron IRB Electronic Submission System
The first chart of additional metrics provides data that indicates the volume of active protocols that HRPP and the IRB maintains. The second chart provides information on the volume of submissions received as well as the volume of submissions processed by the HRPP on a monthly basis for the 2023 calendar year.
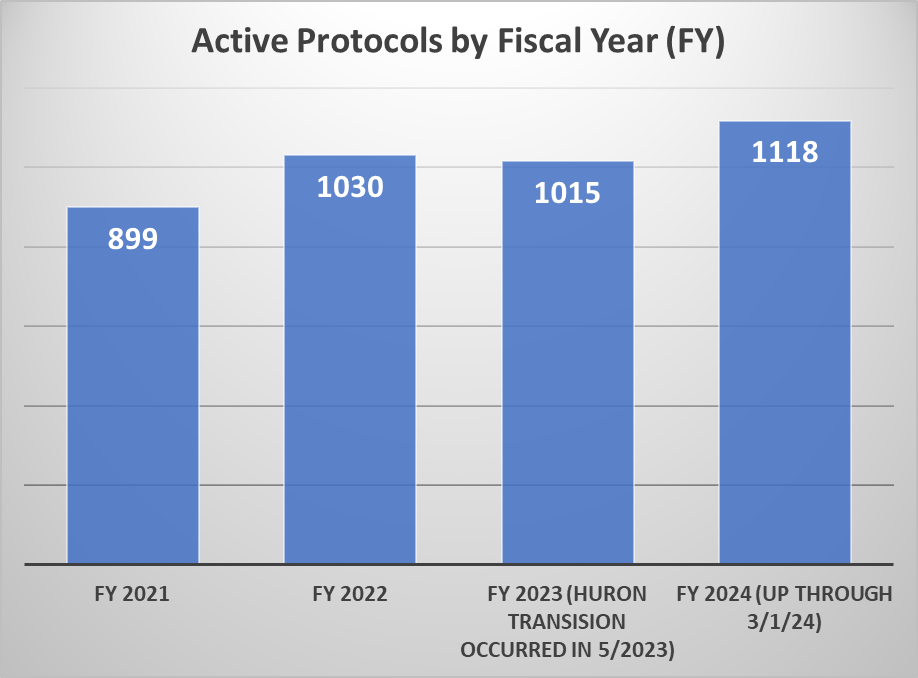
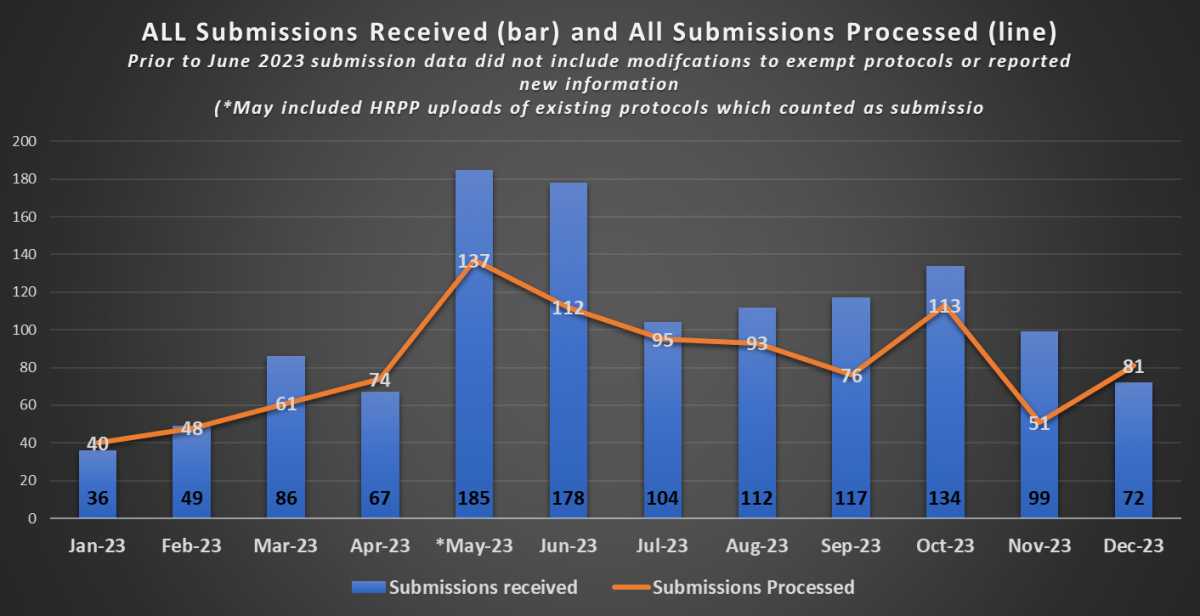
* Through FY 2021 and part of FY 2022, ORI’s Quality Assurance efforts focused on working with investigators to close studies that were no longer active in preparation for transition to the new electronic system. This resulted in closure of 310 protocols thereby accounting for a decrease in the total number of active protocols between FY20 and FY21/FY22.
Protocol Record Keeping, Best Practice Reviews and Sessions
Best Practice Reviews and Session
The QA/QI program offers Principal Investigators (PIs) and their study teams individualized support and guidance to assist in several aspects of protocol maintenance and compliance. This assistance is available to all PIs with a Brown University IRB or HRPP approved protocol. In part, it consists of a one to two-hour meeting which focuses on providing guidance and recommendations related to IRB file organization and maintenance. These sessions may be requested by the PI at any time and, and at the discretion of the PI, a session may be conducted directly with the study coordinator or lab manager. These sessions may also take place:
- at the request of the IRB as a for-cause review, or
-they may be conducted as part of routine QA/QI compliance activities at any time.
Topics covered during a session include a review of compliance issues related to the maintenance of IRB approved study procedures and protocols, consistency with federal regulations and Brown IRB/HRPP policies and procedures. PIs will find this session particularly informative in preparation for external reviews or audits. Best Practice sessions may be scheduled to take place in-person at your research lab or via Zoom. Contact Christiana Provencal for additional information or to schedule a session.
Best Practice Review Checklist
A Best Practice review will utilize a comprehensive checklist as a guide for confirming compliance and identifying areas or topics that may require additional attention or procedural detail.
The checklist is also intended to be used independently by the research team at any time, as a self-assessment tool. PIs and study teams are encouraged to use the checklist periodically during the course of the study. Its use can assist the research team with ensuring protocol compliance, confirming study records maintenance, and providing documentation of internal study assessments, while also self-identifying areas for improvement or gaps in information. Upon completion of the checklist as a self-administered tool, the PI or PI designated study team member is encouraged to reach out to the QA/QI program if they would like additional feedback or recommendations, particularly if gaps or compliance oversights are identified.
