Ground-breaking, creative solutions are developed in an open, honest, and collaborative environment. We strive to create a group dynamic that supports the research, teaching, and outreach goals of each lab member through hard work, excellent utilization of resources, and compassion for each other, the University, and all of humanity.
Our Research and Training Missions:
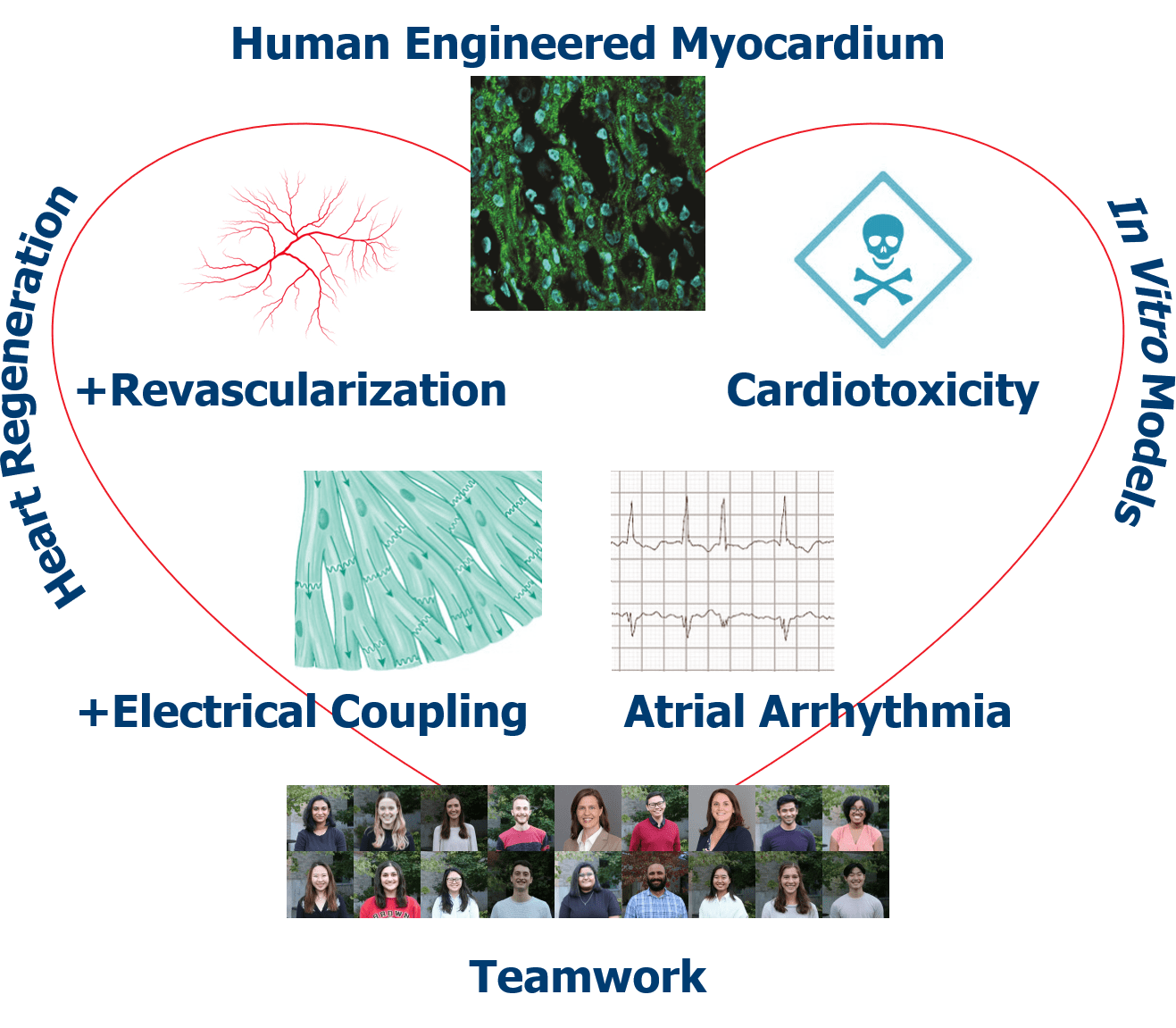
- To passionately pursue cardiac regenerative medicine — from fundamentals of tissue formation and contractility to integration with the host heart — to develop translational therapies for heart disease patients around the world.
- To empower the next generation of scientists and engineers to implement their ideas for improving health and society through attentive mentoring, engaged scholarship, and active inclusion.
Recent Publications:
Soepriatna, A. H., Navarrete-Welton, A., Kim, T. Y., Daley, M. C., Bronk, P., Kofron, C. M., Mende, U., Coulombe, K. L. K., & Choi, B. R., 2023. Action potential metrics and automated data analysis pipeline for cardiotoxicity testing using optically mapped hiPSC-derived 3D cardiac microtissues. PloS one. https://doi.org/10.1371/journal.pone.0280406
Schmitt, P.R., Dwyer, K.D., Minor, A.J., & Coulombe, K.L.K., 2022. Wet-Spun Polycaprolactone Scaffolds Provide Customizable Anisotropic Viscoelastic Mechanics for Engineered Cardiac Tissues. Polymers. https://doi.org/10.3390/polym14214571
Daley, M. C., Mende, U., Choi, B. R., McMullen, P. D., & Coulombe, K., 2022. Beyond pharmaceuticals: Fit-for-purpose new approach methodologies for environmental cardiotoxicity testing. ALTEX. https://doi.org/10.14573/altex.2109131
Schmitt, P.R., Dwyer, K.D., Coulombe, K.L.K., 2022. Current Applications of Polycaprolactone as a Scaffold Material for Heart Regeneration. ACS Appl Bio Mater. https://doi.org/10.1021/acsabm.2c00174
Minor, A.J., Coulombe, K.L.K., 2022. Stimulating Calcium Handling in hiPSC-Derived Engineered Cardiac Tissues Enhances Force Production. Stem Cells Translational Medicine. https://doi.org/10.1093/stcltm/szab002
Soepriatna, A.H., Kim, T.Y., Daley, M.C., Song, E., Choi, B.-R., Coulombe, K.L.K., 2021. Human Atrial Cardiac Microtissues for Chamber-Specific Arrhythmic Risk Assessment. Cel. Mol. Bioeng. 14, 441–457. https://doi.org/10.1007/s12195-021-00703-x
Reid, J.A., Dwyer, K.D., Schmitt, P.R., Soepriatna, A.H., Coulombe, K., Callanan, A., 2021. Architected fibrous scaffolds for engineering anisotropic tissues. Biofabrication. https://doi.org/10.1088/1758-5090/ac0fc9
Bai, Y., Kaiser, N.J., Coulombe, K.L.K., Srivastava, V., 2021. A continuum model and simulations for large deformation of anisotropic fiber-matrix composites for cardiac tissue engineering. J Mech Behav Biomed Mater 121, 104627. https://doi.org/10.1016/j.jmbbm.2021.104627
Kofron, C.M., Kim, T.Y., Munarin, F., Soepriatna, A.H., Kant, R.J., Mende, U., Choi, B.-R., Coulombe, K.L.K., 2021. A predictive in vitro risk assessment platform for pro-arrhythmic toxicity using human 3D cardiac microtissues. Sci Rep 11, 10228. https://doi.org/10.1038/s41598-021-89478-9
