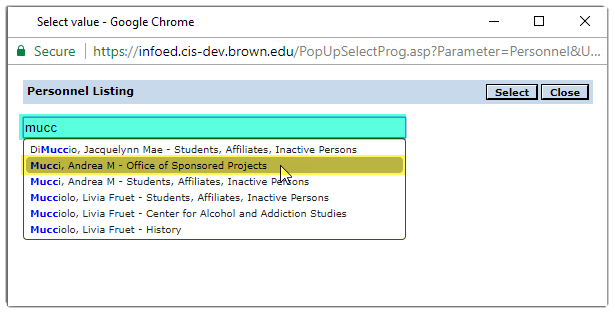
Personnel
Click on the![]() icon to open the Personnel Listing pop up window.
icon to open the Personnel Listing pop up window.
Begin typing the person’s last name in the search field.
Click on the row that contains person’s name.
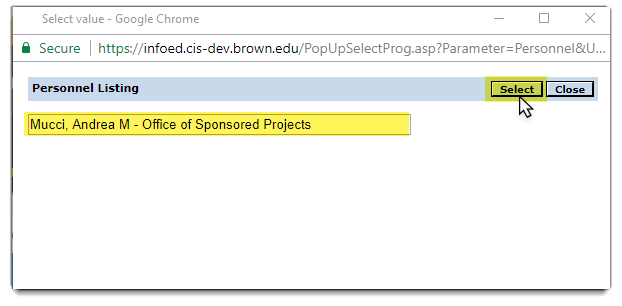
The name will then appear in search field.
Then click the select button  to add person to protocol.
to add person to protocol.
Sponsor (Funding Source)
Click on the![]() icon to open the Sponsors pop up window.
icon to open the Sponsors pop up window.
Begin typing the sponsor’s name in the search field.
Click on the row that contains the sponsor’s name.
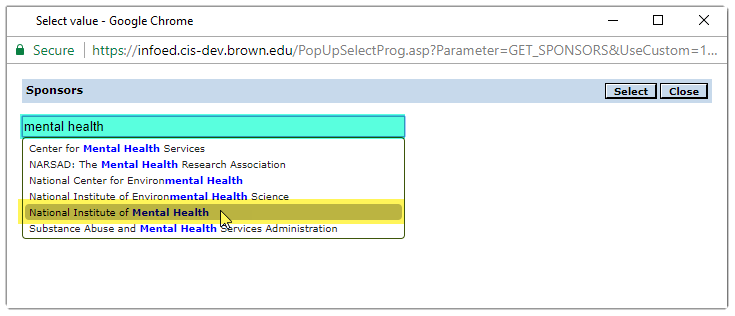
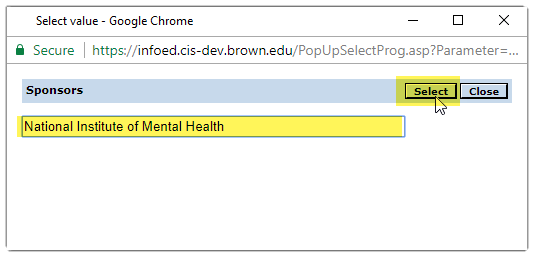
The name will then appear in search field.
Then click the select button 
 to add sponsor to protocol.
to add sponsor to protocol.
Species
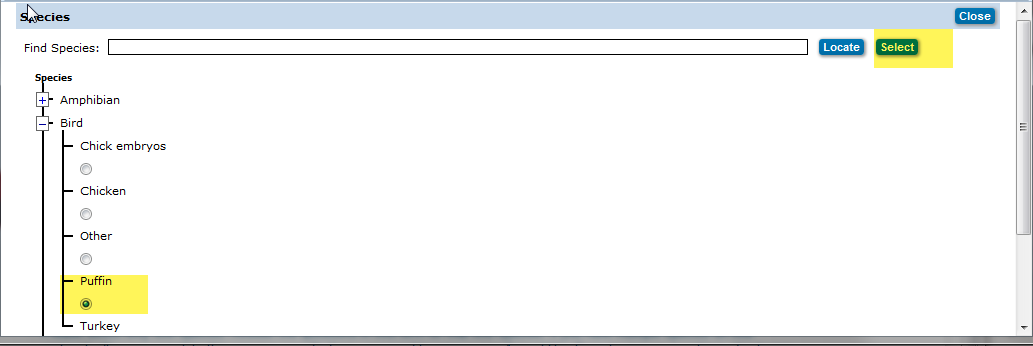
Click on the![]() icon to open the Species pop up window.
icon to open the Species pop up window.
Click on the ![]() next to species category, listing of all species will appear, click on the radio button below the species, scroll back to top and click the
next to species category, listing of all species will appear, click on the radio button below the species, scroll back to top and click the  button.
button.
Species Pop up Window
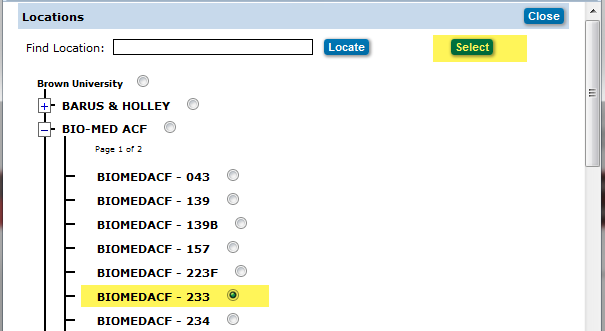
Locations
Click on the![]() icon to open the Locations pop up window.
icon to open the Locations pop up window.
Click on the ![]() next to the building name, listing of all rooms in building will appear, click on the radio button to the right of the room number, scroll back to top and click the
next to the building name, listing of all rooms in building will appear, click on the radio button to the right of the room number, scroll back to top and click the  button.
button.
- Radio button next to building name should NOT be selected.
Locations Pop up Window
Adverse Events
Adverse Event: An instance of unfavorable or unanticipated (not in the approved protocol) signs or outcomes where there is direct harm to animals or personnel. Adverse events include suboptimal well-being (i.e., poor welfare), animal death, disease, distress, or trauma that was not the anticipated result of the approved protocol.
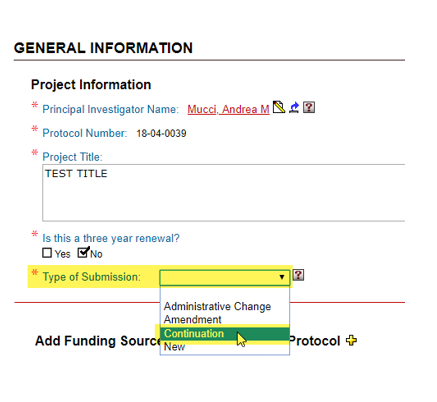
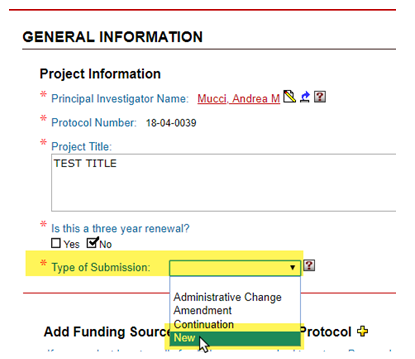
Types of Submission
New
A New protocol covers newly proposed projects, whether they are to be funded externally (e.g., by NIH or NSF) or internally (e.g., using start-up funds, seed funds, via Brown award-granting programs. New protocols are approved for a maximum of 3 years, at which point a De Novo Renewal protocol must be submitted.
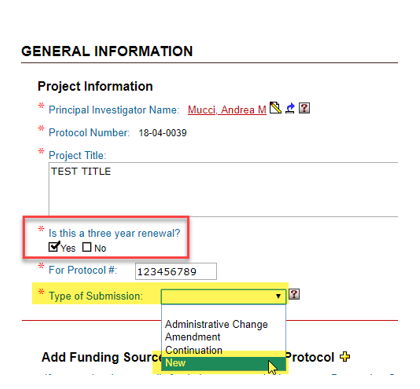
At the “General Information” page, select “Type of Submission” as “New”.
De Novo (Three Year) Renewal
All protocols are set to expire three years after their original IACUC approval date, at which time the project must be reviewed and a new protocol must be submitted.
At the “General Information” page, click “Yes” to “Is this a three year renewal?”. Select “Type of Submission” as “New”.
Administrative Change
Administrative changes are modifications that do not require IACUC review. They are reviewed by the ARC team and typically implemented within 1-2 business days. Administrative changes include: adding / removing personnel; adding removing funding source(s); and changing housing locations.
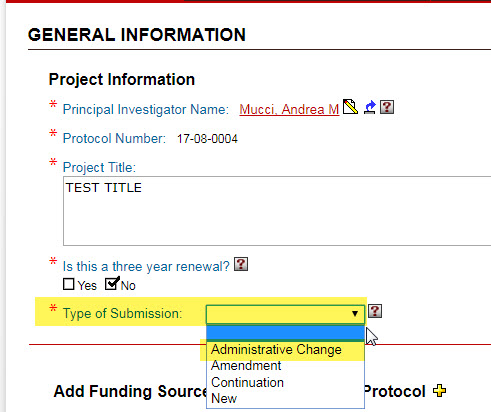
If you are requesting one or more of these changes, please return to the “General Information” page and change your “Type of Submission” selection to “Administrative change.”
Amendment
Amendments are for substantive changes that must be reviewed by the IACUC or via Veterinary Verification and Consultation (VVC). Amendments that may not be handled by VVC must undergo either full committee or designated member review. These typically include (but are not always limited to):
- A change from non-survival to survival surgery
- Any change resulting in greater pain, distress or degree of invasiveness
- A change in study objectives (Note: this may instead require a new protocol.)
- Addition of a new procedure type
- A change from a method of euthanasia approved by the AVMA Guidelines for Euthanasia, to a method that is not approved
- A change that impacts safety of personnel
- A change in Principal Investigator
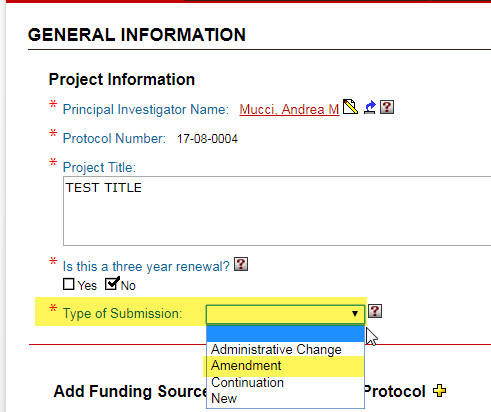
If you are requesting one or more of these changes, please return to the “General Information” page and change your “Type of Submission” selection to “Amendment.”
Continuation
Continuations are required annually for protocols that use USDA-covered species or are for projects funded by the Department of Defense.
At the “General Information” page, select “Type of Submission” as “Continuation”.