Developing Modeling Tools for Examining Vapor Intrusion
Vapor intrusion involves the migration of volatile (and in some cases semi-volatile) contaminants present in the subsurface of contaminated properties into above ground structures. The indoor air concentrations at which these chemicals may pose a human health risk can be extremely low and in some cases on the order of parts-per-trillion. The problem of soil vapor intrusion into structures has been studied for decades, but continues to be a difficult process to properly characterize in the field. The goal of this project is to develop for environmental professionals a computer modeling tool that will reduce the amount of empiricism currently inherent in examining the problem, and to better guide the field studies of potentially problematic sites. The results indicate that soil gas contaminant concentrations by themselves are not necessarily good indicators of a potential vapor intrusion problem; rather the potential for soil gas transport must be considered in conjunction with soil gas concentration data.
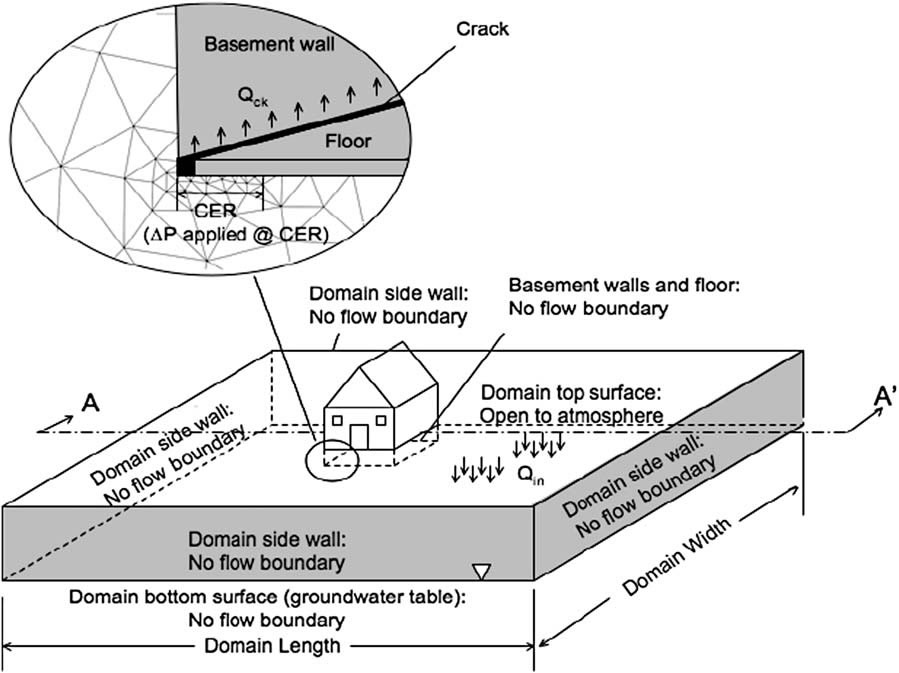
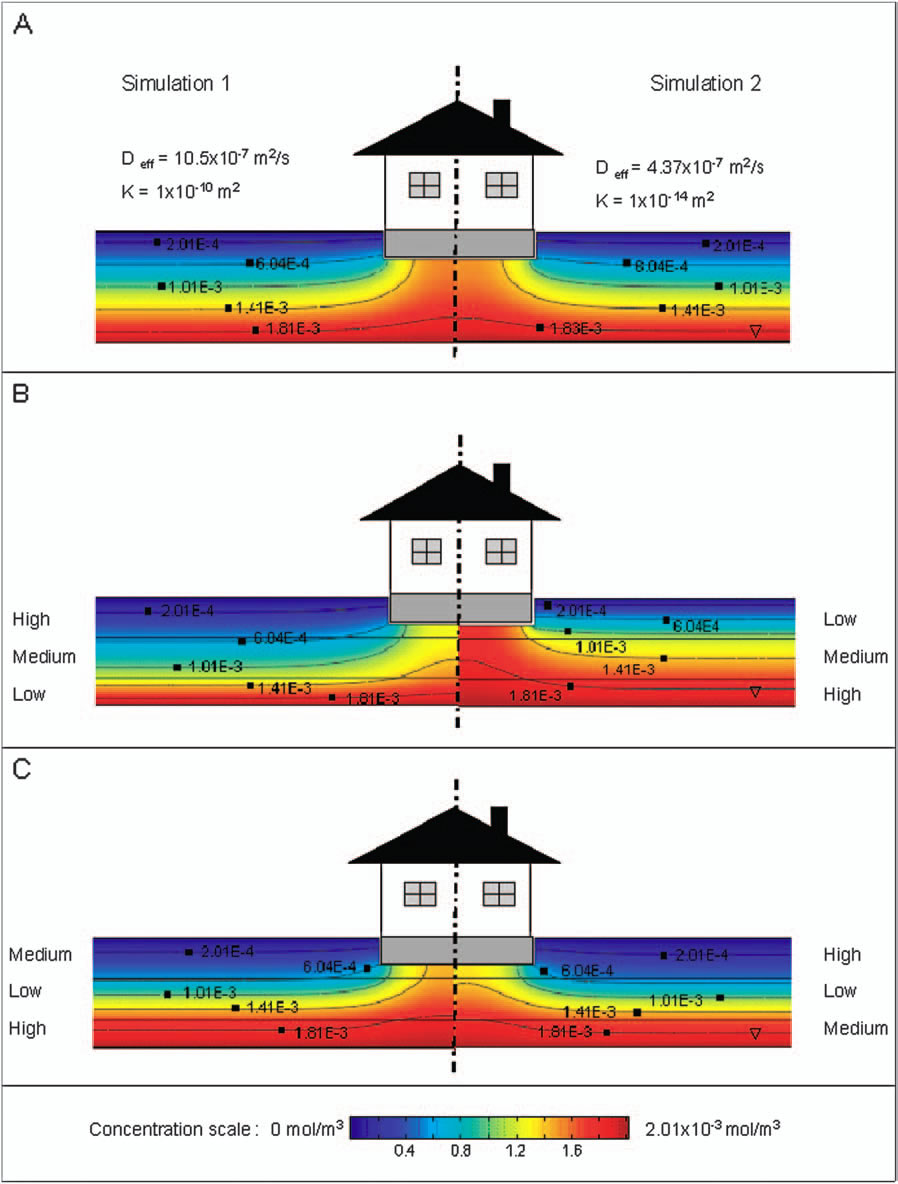
Bozkurt, O., Pennell, K.G. Suuberg, E.M. Simulation of the Vapor Intrusion Process for Non-Homogeneous Soils Using a Three Dimensional Numerical Model. Ground Water Monit. Remediat. 29(1) 92-104 |
Thermodynamics of mixtures of high molecular weight organic compounds
Problem Statement: The knowledge of physicochemical properties of semi-volatile organic substances is essential to understanding the their distribution in the environment. This knowledge is also important to those involved with manufacturing or waste treatment processes of products. Vapor pressure determines the tendency of a chemical to transfer to and from gaseous environmental phases. This property is critical for prediction of either the equilibrium distribution or the rate of change to and from natural sources.
Objectives: PACs generally result from incomplete combustion and many fuel processing operations, and are commonly found as subsurface environmental contaminants at sites of former manufactured gas plants and many other fuel processing facilities. This project aims to experimentally determining the phase behavior and crystal structures of polycyclic aromatic compounds (PACs) and PAC mixtures, and computationally predicting the thermochemical properties and crystal structures of pure PACs and PAC mixtures. The obtained data for PAC mixtures will provide guidance for cleanup of lands and sediments contaminated with mixed PACs.


Subsurface Solid Tar and Non-aqueous Phase Liquids |
Fire Safety
Problem Statement: Brominated flame retardants (BFRs) have been used for more than four decades in electronic equipment and building materials such as circuit boards, capacitors, and textiles to increase their flame resistance. Addition of BFRs is one of the lowest cost ways to meet flammability regulatory requirements. However, there remain many aspects of BFRs that are not well understood. There are the well-known environmental concerns associated with their fate and transport outside of products, and there are always questions regarding how to design better products in terms of retardancy. All of these issues require an improved understanding of properties of these materials, alone and in mixture.
Objectives: The goal of this project is to study the phase behaviors, phase transition energy and thermal degradation of the most intensively used BFRs, i.e. tetrabromobisphenol A (TBBPA) and hexabromocyclododecane (HBCD) –– and three commercial mixtures of polybrominated diphenyl ethers (PBDEs), –– i.e. decabromodiphenyl ether (Deca-BDE), octabromodiphenyl ethers (Octa-BDEs) and pentabromodiphenyl ethers (Penta-BDEs). Such data contribute to both the National Institute of Standards and Technology and the US Environmental Protection Agency databases on this commercially significant class of materials. In addition, this study also providence guidance for BFR selection and design, both as addictive and reactive.
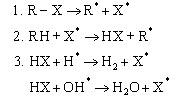
BFRs interrupt the radical chain mechanism of the combustion process in the gas phase (X = Br, Cl):
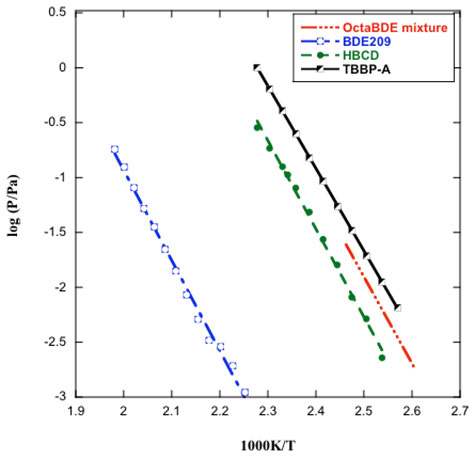
Vapor Pressure of the Most Intensively Used BFRs
|




