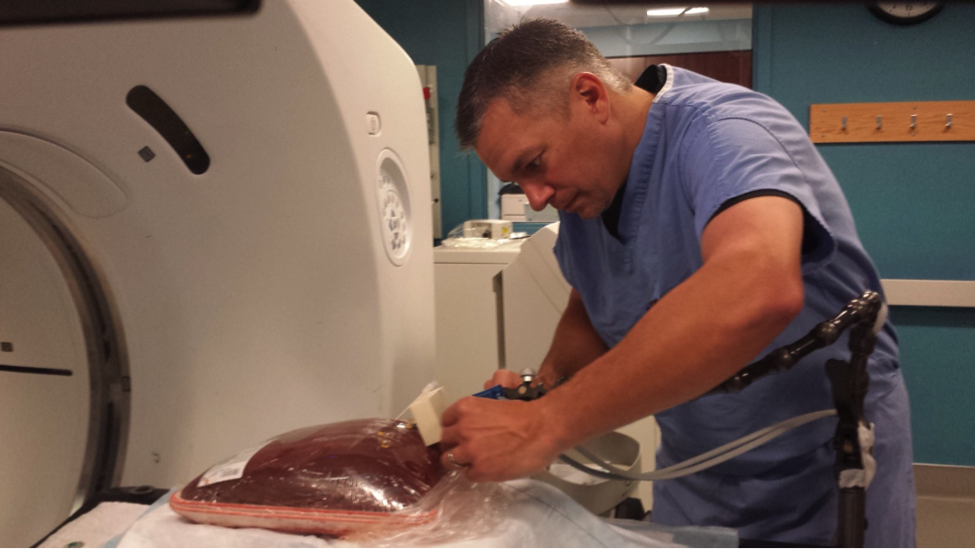
Scott Collins, head CT technologist at Rhode Island Hospital, is inserting a needle into a cow liver. He then takes x-ray's of the liver over time as the needle emits heat to see how the heat is spreading, and changing the tissue. This could help researchers improve heat-based cancer treatments.
When Mrs. Hope Tucker's brother contracted lung cancer, she watched in horror as his unsuccessful surgery to remove the tumor left him tied to an oxygen machine. In the end, it only increased his suffering during his last few weeks. So when she was also diagnosed with lung cancer, she chose a different approach.
Mrs. Tucker chose a treatment where the doctor simply inserts a needle into the tumor site, and the needle kills adjacent cells through heat, cold, or chemicals. This procedure is called ablation. Compared to traditional treatments like surgery, radiation, or chemotherapy, ablation affects a much smaller area, and has fewer side-effects. Whereas the former three treatments are like cannon balls, killing the cancer cells and everything around them, ablation is more like a guided missile.
Doctors have long used ablation to treat benign growths on the skin's surface, or to target precise spots in surgeries. From these procedures, they got the idea that a longer ablation needle can reach and kill tumors deep within the body without cutting the patient open. But the problem was that since they couldn't see these tumors during treatment, they depended on imaging technologies like CT scans and MRIs.
In the 1970's, CT technology became advanced enough to show inside organs. This led to the adaptation of ablation therapy to treat tumors in the 1980's. Heat-based ablation therapy has become the most popular method because its effect size is easier to control than chemical-based methods, and it can reach a larger area than cold-based technology inside the body. The needle emits electromagnetic waves that heat up tissue much like microwaves in the kitchen heat up food. The goal is to heat the tumor to over 140° Fahrenheit, the temperature at which cells begin dying. Less popular methods involve freezing the cells and killing them with chemicals such as ethanol.
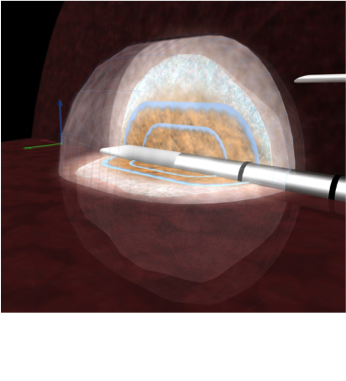
Simulation of how the heat spreads from the ablation needle over time.
Ablation therapy is a minimally-invasive. Unlike traditional surgery, where patients spend days or weeks in recovery, ablation therapy patients can leave the hospital a few hours after their treatment. Since the treatment area is so local, ablation does not wreak havoc on the rest of the patient’s body like chemotherapy or radiation can.
For Mrs. Tucker, a retired activist in animal rights, this is the main benefit of ablation over other treatments. "They can't guarantee it, that it's not going to come back," she said. "But 84, to be able to continue to live my life as I always have. I think it's a blessing." She adds, "I don't know how I would've done if I’d selected the operation. I think it'd have taken an awful lot out of me."
Initially tested on liver cancers, ablation was gradually adopted for other organs such as the kidneys and lungs. Dr. Damian Dupuy, a doctor at Rhode Island Hospital, performed the first ever lung tumor ablation in 1998. He had seen the initial adoption of ablation for cancer treatment during his residency at Massachusetts General Hospital, where he was struck by its potential to revolutionize patient care.
During an interview, he stressed that ablation could preserve healthy tissue while treating cancer much better than anything else available right now. "Eventually you're going to run out of real estate,” he says. “You're not going to have enough liver left. Why not do something that spares large chunks of liver?" Especially in the kidney, it's important to conserve as much of the organ as possible. Patients with one tumor have a 15-20% of getting another kidney tumor in their lifetime. With surgery, doctors would cut off whole lobes of the kidney, but with ablation, they can have finer control on the treatment area.

Dr. Damian Dupuy, in the operating room, explains his plan for the ablation treatment to three researchers as nurses prepare the patient and ready the machine.
The only problem is that ablation doesn't work as well.
When Dr. Dupuy performs the procedure, he first scans the patient to determine where the tumor is and inserts the needle as near the tumor as possible, preferably into it. He then takes another image to see if the needle is in the right place. If not, he would adjust the needle placement and scan again. After getting the right placement, he turns on the generator and waits for a period of time to reach to desired treatment volume. Then he turns the needle off and scans the patient again to check that everything is truly killed. If not, then he performs a "touch up," repositioning the needle and ablating the remaining section. Since he can't see the tumor, the scans are the only indication of whether the treatment was successful.
This means that ablation therapy's effectiveness depends on the imaging technology to see the tumor. Although doctors do observe changes in the images after treatment, the reason for the change is not clear. They assume that any tissue that looks different has been heated up sufficiently for a long enough period of time, but the evidence for this is more anecdotal than scientific. For all we know, the change could be happening at 50°, just under the treatment temperature. With surgery, there is no question of whether a tumor has been cut. Doctors can test the tissue directly. The tumors can also present challenges. Solid, regularly shaped tumors show up well on images but if the tumor’s shape is ill-defined or the tumor has thin tendrils branching off it, there is the possibility that the tumors are bigger than they appear. The missed cancer cells could lead to recurrence in the same area, called local recurrence. Today's ablation technology has a very limited effective treatment zone, which makes under-treatment a serious concern. Doctors have to know in advance to use more ablation needles if the tumor is bigger. While missed offshoots are also a problem for surgery, doctors generally have to cut off a much larger volume to reach the tumor anyway. This gives them a larger safety margin.
To combat this, doctors need to know exactly what type of tumor it is, and how much safety margin they need to treat around it to account for all the offshoots. Ideally, after doctors define the volume they want to treat, they would know the exact power setting and time they need to achieve that volume. But since different tissue heats up differently, and blood constantly moves heat around the body, an accurate prediction of the treatment zone remains unachieved, but it is an active area of research.
As a result, ablation has a fairly high recurrence rate compared to surgery. The vendors' specifications for the needles are generally derived from tests on dead cow livers, which heat up completely differently than a human liver in a living body. The cost of not having an accurate model is insufficient treatment. Two years after her first ablation, Mrs. Tucker had to return for a second procedure to treat a local recurrence. Fortunately, she has been living cancer-free since then.
Looking at post-procedural images, Scott Collins, the chief CT technologist at RIH said, "Tumor is sticking out of what we're defining as the treated zone." This observation led to an on-going study at RIH to review whether they could identify ablation cases where tumor is sticking out, and whether those cases really had higher local recurrence rates. They hope to identify and incorporate features from these cases to better predict the treatment zone in the future.

Mr. Garron Deshazer waits outside the MRI facilities for his test to ablate a cow liver to finish. Behind him, two students are processing the scans as they come off the machine.
Garron Deshazer, a physics PhD student at University of Rhode Island working at RIH, is creating a better model of how heat spreads at different needle settings and different tissues throughout the treatment period. The type of tumor, the tissue surrounding it, and the distance from major blood vessels all affect how heat spread. After he creates these models, he ablates cow livers and then slices them to see if the heating happened as he predicted. This will remove some uncertainty of whether the changes we see in the scans match up with actual treatment zone. He says that if this is successful, he plans to begin live animal tests with pigs. The final test would be in humans.
For human tests, patients scheduled for surgery will first be treated with ablation therapy, and then the surgery will be performed as planned. The doctor will only heat the tissue that will be removed in the surgery anyway, so that these tests of ablation therapy will not harm the patient. Mr. Deshazer can then slice up the tumor and see the effects of ablation in a live human.
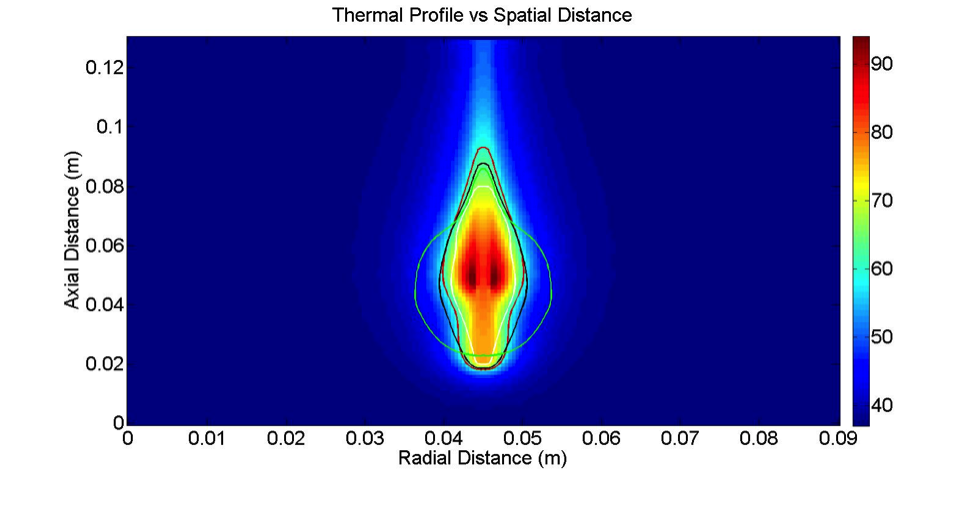
The background colors show the temperature of the cow liver immediately after ablation therapy. The colored lines to show predictions of the treatment zone under different settings, and researchers compare the two to see which predictions were the most accurate.
But one problem preventing the widespread use of ablation is the lack of data supporting its success rate. Because ablation therapy has less data to support its effectiveness than other treatments, doctors are hesitant to recommend it. Without doctors' recommendation, patients don't undergo ablation and there is no data.
Today, ablation is a treatment of last resort. It is reserved for older patients, patients with complications from other diseases, and recurrence cases. These are people who do not have many other options. Although Dr. Dupuy says that there is emerging data to suggest that ablation is as effective as surgery in small kidney and liver tumors, the lack of eligible patients limits the scope of the study. Since doctors generally perform ablation on elderly, or already sickly patients, there is little direct data to show that it is effective for young and otherwise healthy patients. Since medicine is a very conservative field, doctors continue to recommend more aggressive but proven treatments to patients who can undergo them.
But just because a patient can tolerate a more invasive treatment doesn't mean it's necessarily the best choice for them.
Although Mrs. Tucker was a good candidate for ablation, her doctor didn't even offer it to her as an option. He strongly recommended that she undergo surgery because it had a better long term survival rate. But she was concerned about surgery because of her brother's experience. Not satisfied with the option her doctor gave her, she turned to other patients for advice.
Coincidentally, her neighbor was diagnosed with lung cancer a few years earlier. He followed his doctor's recommendation to get surgery. Not only did the treatment completely take over his life, it was unsuccessful and the cancer came back almost immediately. Since radiation and surgery don't generally work a second time, his daughter, who worked at RIH and knew Dr. Dupuy, encouraged him to try ablation therapy instead. Although ablation also failed to beat his cancer, he has been able to keep it under control with repeat treatments. Most importantly, "He looks great. He's out and about living his life," says Mrs. Tucker.
But doctors are generally wary of new treatments, especially ones that lead to worse long-term success rate. "You usually choose the most aggressive approach that has the most data supporting the use," in choosing a cancer treatment, Dr. Dupuy says. But this conservative approach is a barrier to innovation. We expect changes in medicine to be slow and we want doctors to be careful. But this no longer seems beneficial when patients need personal connections to even find out what their options are.
Patients would also like to have a say in their treatment. While doctors can save lives, only patients can decide on the quality of life they want. Although Mrs. Tucker fully understands that surgery has better chances of beating cancer in the long run, she said that at 84, she was more concerned with the quality of her life after the treatment. "I really feel that it should be offered as an opportunity for older people, which I don't think is being done," she says emphatically. "I really feel like I'd done the right thing."