Energetic Materials
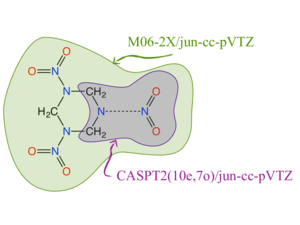
Our goal is to predict the decomposition kinetics of energetic materials, primarily caged polynitroamines, such as RDX, HMX, CL-20, and others.
Our initial effort focused on the decomposition of RDX in the gas phase. To capture N-N bond fission accurately, we used variable reaction coordinate transition state theory. However, pure CASPT2 calculations were too expensive, so we adopted the embedding scheme of Miller and Manby; the multireference calculations were embedded in DFT calculations. The results are in excellent agreement with the available literature data.
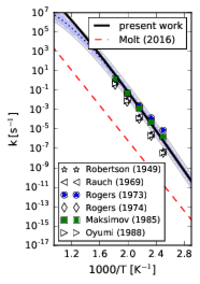
These results can be found in our recent article in the Proceedings of the Combustion Institute.
More recent efforts have focused on applying coupled-cluster methods to larger compounds, such as CL-20 and octonitrocubane. We're also modifying the open-source code RMG to predict the complete decomposition mechanism for these compounds. We are currently developing a microcanonical master equation for molecular crystals, which will combine variational transition state theory with an energy transfer model.
