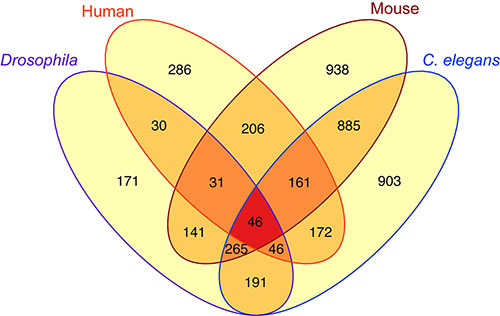
Researchers found 46 genes shared by worms, flies, mice and humans that are all targets of FOXO proteins, which are important operators in the molecular mechanics of aging and longevity.
Courtesy of Ashley Webb
PROVIDENCE, R.I. [Brown University] — Whether a creature is a worm, a fly, a mouse, or a human, death inevitably awaits. And not only do these organisms share a common fate, but also, according to a new study, they may share some of the specific mechanisms of mortality. The researchers found that in all four species, there are 46 genes regulated by the same family of “FOXO” proteins known to be central in aging and longevity.
“We identify for the first time the set of direct FOXO targets that are conserved across evolution,” wrote the scientists led by Ashley Webb, assistant professor of molecular biology, cell biology and biochemistry at Brown University. The study appears in the journal Aging Cell.
The 46 genes targeted in common across that wide range of species — those that have been “conserved” throughout evolution — have roles in metabolism, DNA repair, and other processes important in aging. Previous research has shown, or at least suggested, that manipulations and mutations in FOXO genes can extend or reduce lifespan in each organism, even though some diverged from each other on the proverbial tree of life about 500 million years ago.
In specific cells or across whole tissues, FOXO affects many more genes in individual organisms than the 46 found in common across all four species. A key goal of the new study, therefore, was to determine which genes probably matter the most in aging and longevity.
“Several studies have been published reporting thousands of FOXO targets,” Webb said. “These studies leave us with a huge unanswered question: Which of these many targets are the crucial effectors of FOXO in aging and cellular homeostasis? It would take years to test each target one by one, so we asked which of the targets have been conserved through evolution, reasoning that the most important targets would be highly conserved.
“This study identified genes that are known to be FOXO targets, which is reassuring,” Webb said. “But we are also excited because we found several novel targets that were not identified previously and are conserved in all species examined.”
By revealing dozens of known and previously unknown gene targets that FOXO proteins affect across species, the study, which Webb conducted with Anshul Kundaje and Anne Brunet at Stanford University, provides new guidance on where the biology of aging field might look more closely.
The data also could help researchers develop hypotheses about FOXO in humans, given what they have in common with the easier-to-study organisms.
“Based on our findings in the mouse, we believe that FOXOs are likely to have tissue-specific targets in the human, suggesting that modulating FOXO’s activity would have very different effects on different tissues,” Webb said. “This is important because it suggests that interventions that affect lifespan will have dissimilar effects on different tissues.”
Ultimately, developing ways to use knowledge of FOXO proteins to positively affect health and longevity in humans is a key goal, Webb said.
“It is essential that we understand how central regulators of aging function in the normal context, and to what extent their function is altered during aging or under conditions of stress, leading to tissue deterioration,” Webb said. “In other words, if we want to enhance tissue function in the elderly, we need to first know how a normal healthy cell functions, and why it becomes dysfunctional with age.”
Funding for the study came from the National Institutes of Health (grants: AG036695, P20GM103430) and the Alfred Sloan Foundation.