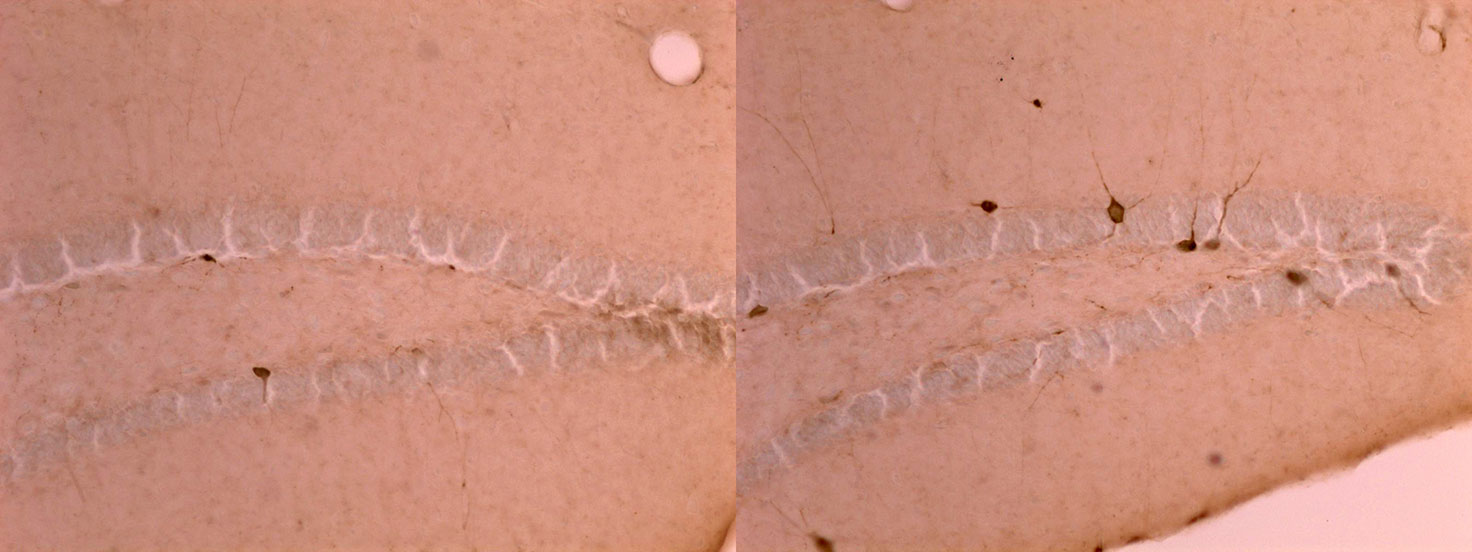
By 28 days of age, many dark brown parvalbumin interneurons had developed in tissue from a stress-exposed mouse on the right, but they had yet to develop in the unstressed mouse on the left. Bath et. al./Brown University
PROVIDENCE, R.I. [Brown University] — Intuition is all one needs to understand that stress in early childhood can create lifelong psychological troubles, but scientists have only begun to explain how those emerge in the brain. They have observed, for example, that stress incurred early in life attenuates neural growth. Now a study in male mice exposed to stress shows that a particular region, the hippocampus, hits many developmental milestones early — essentially maturing faster in response to stress.
The findings, the first to track and report signs of stress-related early maturation in a brain region throughout mouse development, may lend some neuroscientific credence to the expression that children facing early adversity have to “grow up too fast.”
Lead author Kevin Bath, assistant professor of cognitive, linguistic and psychological sciences at Brown University, said he became curious about whether some brain regions were maturing faster because he and other researchers had made observations in humans and rodents all suggesting that certain traits — such as fear-driven learning and memory, sexual development and neural connectivity among some brain regions — were accelerated, rather than stunted, after early life stress (ELS). Some of these qualities, particularly memory and emotion regulation, involve the hippocampus.
“There were a number of different indicators that [early maturation] might be happening,” Bath said. “We wanted to carefully assess this and look at a number of different markers of not only growth, but also maturation of these animals, and to measure it not only at the behavioral level but also at the neuromolecular level.”
The study, co-authored by Brown graduate students Gabriela Manzano-Nieves and Haley Goodwill, appears online in the journal Hormones and Behavior.
Experiment in early life
The experimental stress introduced to the mice in the study was a period of fragmented maternal care — a condition comparable to one that might affect a child growing up in an economically challenged, single-parent household, for example.
At four days of age, pups and their mothers were moved from standard cages to ones where the materials available to the mother for nest building were inadequate. Food and water remained plentiful, but the mother responded anxiously and would frequently depart to search for anything that might work as nesting material. Pups therefore received less consistent and attentive care from their harried and distracted mothers than experimental controls who were never moved from standard cages. After just a week of exposure to this manipulation (a significant span of time for mice who mature from birth to adulthood in just eight weeks), the mice returned to cages with everything they needed.
By then, however, the effects of the ELS were underway. Bath and his co-authors made several measurements in mice aged four to 50 days (when mice reach young adulthood) to track how development in the hippocampus varied between mice with ELS and the unstressed controls.
What they found — from counting specific populations of cells, to measuring behavior, to gene expression — was that the hippocampus appeared to mature significantly faster in the ELS mice during their seven weeks from birth to early adulthood.
Based upon measures of gene expression and counting cells, the team saw that parvalbumin interneurons developed about a week early, attaining an abundance by day 21 in ELS mice that was not seen in control mice until day 28. In other measurements, the teams saw that developmental changes in synaptic receptor subunits that are important for developmental changes in learning occurred about nine days ahead of schedule. They also found that myelination, a key developmental process for neural communication, also started more than a week early.
They also looked at a behavioral trait controlled by the hippocampus. Mice can be conditioned to associate shocks with a particular location, such that they will freeze in fear when they come back to that place. But for about a week during the maturation of the hippocampus, that freezing behavior temporarily disappears, Bath has found. In the new study, he saw that this was still the case, but the temporary suspension of the fear response happened a week earlier in ELS mice than in control mice.
Also, in their study, the team confirmed the findings of previous researchers showing overall reduced neural growth.
A developmental tradeoff?
The results showing reduced growth but faster hippocampus maturation appear to support an evolution-based hypothesis that mice — and perhaps people, too — interpret ELS as a cue to adapt brain development to match a world where long-term survival seems unlikely, Bath said.
“In the case of development, the stress may be providing a signal about the hospitability of the environment,” Bath said.
Rather than invest for the long run in optimally refined systems in the cortex for learning rules and suppressing emotional responses, mice may instead invest in accelerating the maturation of more primal systems, such as the hippocampus, to support short-term priorities. The priority, Bath speculates, becomes racing to survive long enough to reproduce at least once.
“The evolutionary push is for you to pass on your genes,” Bath said. “We hypothesize that stress drives a reallocation of developmental resources from development of the full brain to development of limbic structures that are important for reproduction.”
To know for sure — and to get hints about how mental health practitioners can help people who have experienced early life stress — much more work is needed, Bath said. He’s pursuing several lines of research including studying female mice, given observations that females are twice as prone as males to develop problems in response to stress, and that human girls who have experienced early life stress undergo menarche earlier. He’s also tracking the behavioral implications of accelerated maturational traits (e.g. the early abundance of parvalbumin interneurons), measuring whether maturation is accelerated or delayed in other areas in the brain, and looking at the genetic mechanisms underlying the accelerated maturation to understand why some humans are resilient to ELS exposure.
The Brown Institute for Brain Science, Robert and Nancy Carney and the Brain & Behavior Research Foundation funded the research.