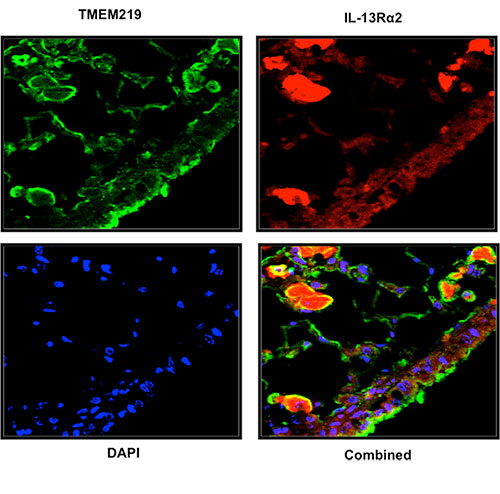
Fluorescently stained Images show how the proteins TMEM219, IL13Rα2, and closely colocate in tissue. DAPI, in blue, marks cell nuclei.
Lee et. al./Brown University
PROVIDENCE, R.I. [Brown University] — The more proteins that prove essential in the pathways of disease, the more targets drug makers and doctors have for intervention. That’s why its potentially important that a new study reports a key role for the protein TMEM219 in pathways that appear to be central to a variety of diseases in the lung.
The new results, published in Nature Communications, show that in many cases transmembrane protein 219 (TMEM219) allows the protein interleukin 13 receptor alpha 2 (IL13Rα2) to signal disease-related pathways when it is stimulated with a third protein, chitinase 3-like-1 (CHI3L1). Elevated levels of CHI3L1, which normally protects against lung injury and promotes tissue repair, are associated with idiopathic pulmonary fibrosis, the spread of cancer in the lung, and asthma.
“CHI3L1 goes up in a number of diseases but for it to do its thing it has to go up in the setting of an appropriate receptor, or receptors, to mediate its effects,” said Dr. Jack A. Elias, senior author of the study and dean of medicine and biologic sciences at Brown University. “Knowing what its receptor or receptor complex is and knowing how it is regulated in that setting plays a critical role in determining if you get beneficial or pathologic effects of CHI3L1.”
Elias and colleagues at Brown and Yale University have been tracking CHI3L1’s role in disease for years. In 2013 they found that it interacts with IL13Rα2. Now they’ve found many cases in which those proteins work as a trio with TMEM219.
Tracking TMEM219
In several experiments the researchers showed that the proteins were closely linked and that their three-way interaction is essential to the functioning of the disease-related pathways. For example, they found that the proteins not only can be found in the same places within cells, but also that they physically bind.
In human cells that line the airways of the lungs they showed that blocking expression of TMEM219 substantially reduced the expression of a growth factor protein that they had known to be regulated by CHI3L1 and IL13Rα2. Similarly, they showed that TMEM219 was a necessary part of the trio in activating the signaling of other key enzymes in human and mouse cells.
In later experiments, the team looked directly into disease-related pathways and found that with TMEM219 blocked or missing, the pathways did not work as they had seen before in their research on CHI3L1 and IL13Rα2. For instance the proteins protect cells — with fatal overzealousness in the case of pulmonary fibrosis — from oxidant-induced death and injury, but they couldn’t as effectively without TMEM219. The researchers also showed that without TMEM219, CHI3L1 and IL13Rα2 could not provide as welcoming an environment for the spread of melanoma to the lung as they normally do. When they blocked or removed TMEM219, cancer spread in mouse lungs was significantly decreased.
In addition, the team showed that TMEM219 promoted the binding between IL13Rα2 and the interleukin 13 (IL-13) protein, which reduces IL-13’s effects in the body. Exaggerated IL-13 induced tissue responses are characteristic of diseases like asthma and allergy, Elias said.
They also found a signaling pathway in which TMEM219 wasn’t necessary, suggesting that there are specific cases where CHI3L1 and IL13Rα2 either work just which each other or with other third proteins instead.
Targeting TMEM219
The overarching goal of Elias’s research is to find ways of safely and effectively manipulating these pathways to fight lung diseases. His group has already been testing antibodies against CHI3L1 and IL13Rα2 in mice. Since learning about TMEM219’s role in many of the same pathways, the lab has developed one against that protein, too.
As testing continues, he said, it may turn out that manipulating TMEM219, or coming up with a way to block its binding to IL13Rα2, may prove to be the most promising avenue to an eventual treatment.
“Let’s say you want to block the functioning of a receptor [in a pathway],” said study lead author Chang Min Lee, research assistant professor of molecular microbiology and immunology. “You don’t know what the consequences will be if you block IL13Rα2 and you don’t know what the consequences will be if you get rid of TMEM219, but if the pathway you are trying to block requires both proteins you have the ability to choose between the proteins in making a drug that blocks the receptor. You can pick the blocking approach that has the lowest toxicity for the patient.”
In addition to Elias and Lee, the paper’s other authors are co-senior author Ghun Geun Lee, Chuan Hua He, Adel Nour Yang Zhou, Bing Ma, Jin Wook Park, Kyung Hee Kim, Charles Dela Cruz, Lokesh Sharma, Mahmoud Nasr and Yorgo Modis.
The National Institutes of Health (grants: R01HL093017, U01HL108638, R01HL115813), the Korea Drug Development Fund (KDDF-20132-11) and the National Research Foundation of Korea (NRF-2012R1A6A3A03040533) funded the study.