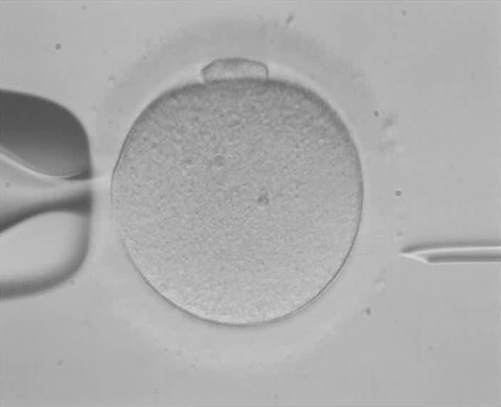
PROVIDENCE, R.I. [Brown University] — The healthy development of an embryo created through in vitro fertilization (IVF) depends on whether most, if not all, of the cells have the proper number of chromosomes. With pre-implantation genetic screening (PGS) technology, doctors can, in principle, spot-check chromosome count before choosing which embryo to implant in the mother. In a new article, however, scholars at Brown University and the University of Washington report that PGS has serious limitations that can only be overcome with more human embryo research, even as they acknowledge the controversy surrounding that research.
What doctors and hopeful parents want to see in PGS is 46 chromosomes — two pairs of 23 — a normal state of affairs called “euploidy.” An abnormal number, or “aneuploidy,” could signal a fatal flaw in early development. In 2013 in the United States, more than 15 percent of IVF pregnancies ended in miscarriage, often because of aneuploidy, wrote Dr. Eli Adashi, professor of medical science and former dean of medicine and biological sciences at Brown, and Rajiv McCoy, a genome sciences postdoctoral fellow at Washington. The miscarriage rate rises quickly with maternal age, as does the rate of aneuploidy.
Hoping to prevent a bitter loss, a growing percentage of infertility patients using IVF have turned to PGS. But as Adashi and McCoy wrote in the journal EMBO Reports, PGS has yielded mixed results. Sometimes it has predicted the doom of embryos that became healthy children, and in the small studies conducted so far, there has been mixed evidence that its use leads to a greater likelihood of a successful pregnancy.
“The impact of PGS on the outcome of assisted reproduction remains uncertain,” they wrote.
Tricky biology and ideology
The problem with PGS, Adashi and McCoy wrote, stems from how little doctors and scientists really know about early embryo development, which is a complex process. There are two main sources of aneuploidy — the original cell division that creates an egg cell, called meiosis, and the division of cells in the growing embryo, called mitosis. The first cause, because it occurs in one of the two sex cells that form an embryo, is especially serious and is known to increase with maternal age. Errors in mitosis will affect some but rarely all the cells in an embryo.
In most applications of PGS, doctors sample genetic material from several cells on the outer edge of a five-day old embryo, called a blastocyst. If that yields evidence of aneuploidy, the test usually still can’t discern whether it’s meiotic, in which case all cells could be affected, or mitotic, in which only a few might be (creating a “mosaic” of ploidy).
Among the many things doctors don’t know is what ploidy status they would find if they could safely look elsewhere in the embryo, including its inner cells. Finally, they don’t understand yet why some mosaic embryos will succeed and others will not.
“Such insights may improve the diagnosis and selection of healthy embryos through PGS and hopefully will lead to the development of new technologies,” Adashi and McCoy wrote.
But human embryo research remains controversial in many places around the world, including the U.S., they acknowledge. Public funding, and sometimes the research itself, is often prohibited.
They conclude with a call to accelerate research.
“This state of affairs hampers the acquisition of new insights into the intricate process of early human development,” they wrote. “More importantly, translational breakthroughs intent on improving infertility care are being delayed. Patients afflicted with infertility deserve better.”