PROVIDENCE, R.I. [Brown University] — Tree-like branching structures are everywhere in the human body, from the bronchial system in the lungs to the spidering capillaries that supply blood to the extremities. Researchers have long worked to understand the cellular signaling needed to build these intricate structures, but new research suggests that simple physics may play an underappreciated role.
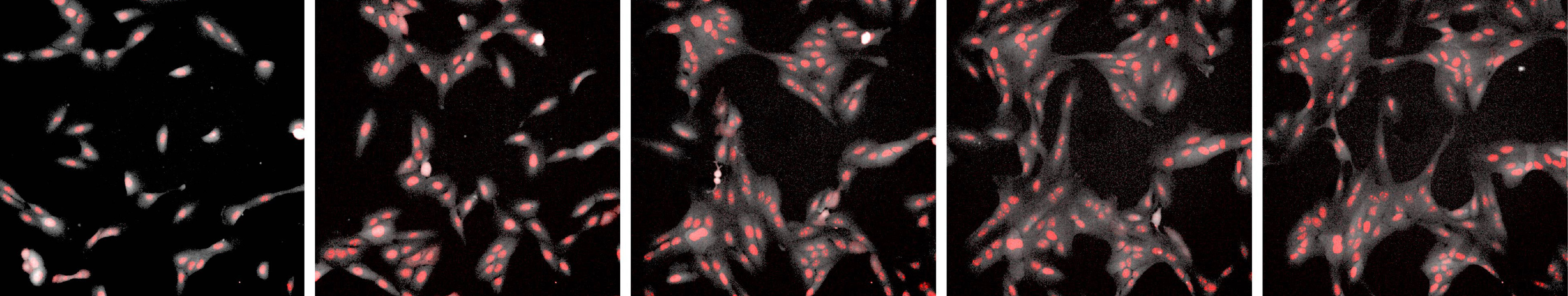
The research, published in the Proceedings of the National Academy of Sciences, shows that under certain circumstances, human epithelial cells behave according to the rules of diffusion-limited aggregation, a theory explaining the self-assembly of particles suspended in a liquid. The study showed that cells cultured at sparse densities and low amounts of growth factor arrange themselves in fractal-like branching clusters. The fractals (patterns in which tiny subsections look the same as the whole) have nearly the exact dimensions predicted by diffusion-limited aggregation.
“What’s remarkable to me is that you have this elegant physical theory that describes the aggregation of sticky particles moving randomly and also explains the behavior of migrating cells,” said Ian Y. Wong, an assistant professor in Brown University’s School of Engineering and senior author on the paper. “In actuality, cells are complicated entities with many ways to send and receive signals, yet in this case they seem to follow the same rules as much simpler, non-living particles.”
The findings could offer insights into the formation of branching structures in the body as well as other key biological processes, said Susan E. Leggett, currently a postdoctoral researcher at Princeton University and the study’s lead author.
“We think this could potentially tell us something about tissue formation, wound healing, cancer invasion and other processes in which moving cells organize into multicellular tissues,” said Leggett, who performed the work as a Ph.D. student in pathobiology at Brown.
When she started the research, Leggett was actually thinking about a very different question. She wanted to learn more about how cells behave when they’re on their own, away from the influence of their neighbors. She’s interested in a process known as the epithelial to mesenchymal transition (EMT) in which clustered epithelial cells transition into more motile mesenchymal cells. The transition tends to happen when cells are isolated from each other.
“My original motivation for seeding cells in these low-growth-factor conditions was to maintain them in a low-density environment so we could study EMT,” Leggett said. “We didn’t anticipate that the cells would undergo this aggregation, which is almost the reverse process.”
The cells in the low-density cultures moved around randomly until they came into contact with another cell, at which point the cells stuck together and stopped moving. Over the course of a few days, the clustered cells assembled themselves into long branch-like structures that were “strikingly reminiscent of fractal-like structures associated with diffusion-limited aggregation,” the researchers wrote.
Further analysis showed that the fractal dimension of cell clusters (a measure of how densely packed the branches are) came out to around 1.7, which is precisely the fractal dimension measured experimentally and predicted theoretically for the diffusion-limited aggregation of particles.
The fact that the cells formed structures so similar to that of inanimate matter could be helpful in understanding how and why branched architectures are so prominent in biology, the researchers said.
“An improved understanding of these collective behaviors may further enable new design rules for programming cells to assemble into larger tissue architectures,” Wong said.
The work was supported by the National Institutes of Health (T32ES007272, P30GM110759, R21CA212932). Other co-authors on the paper were Zachary J. Neronha, Dhananjay Bhaskar, Jea Yun Sim and Theodora Myrto Perdikari.