PROVIDENCE, R.I. [Brown University] — The room is bright and warm — a mild mid-70s year-round — redolent of algae and burbling water. It sounds like a tropical oasis on a remote island. In reality, it’s a laboratory on the Brown campus in Rhode Island, home to a breeding colony of genetically engineered zebrafish.
Jessica Plavicki, an assistant professor of pathology and laboratory medicine at the University’s Warren Alpert Medical School, uses the embryos produced by the adult fish to study developmental genetics — how genes control normal growth — and developmental toxicology — how environmental factors interfere with normal growth and development.
Specifically, Plavicki is investigating how toxic manufactured chemicals — called toxicants — affect brain and heart development. In some cases, her team exposes zebrafish embryos to contaminants found in water supplies to study the impact of those toxicants. They’re particularly interested, she said, in perfluoroalkyl and polyfluoroalkyl substances (PFAS), a group of contaminants of emerging concern that includes byproducts from manufacturing nonstick cookware and waterproof fabrics as well as residue from firefighting foams. Often, they use targeted genetic tools to tweak molecular pathways disrupted by toxicants.

Plavicki’s research is based in zebrafish, yet its implications are widely applicable. Fish may have fins instead of feet and two heart chambers instead of four, but the molecular signals that orchestrate development remain the same in both fish and humans.
“In my lab, we’re interested in understanding how exposure to contaminants can impact the development of the brain and the cardiovascular system,” said Plavicki, who is affiliated with Brown’s Carney Institute for Brain Science. “And we’re really interested in this intersection between different organs — the interaction between cardiovascular health and brain health.”
Attracting and supporting early-career researchers like Plavicki, whose innovative work cuts across multiple areas of focus, is the bedrock of enhancing the impact of Brown’s top-tier research enterprise. And as critical as establishing a new research laboratory is for advancing science, it is no small undertaking.
Launching a lab
Since she arrived at Brown in 2016, much of Plavicki’s work has been focused on establishing the colony of genetically engineered zebrafish and ensuring that each strain of fish has the correct genetic changes — specific cells triggered to glow, or particular molecular signals turned on or off.
Before she could establish the colony, the University had to build a specialized facility to house the zebrafish. This state-of-the-art tropical setup includes racks of fish tanks, two separate water filtration and quality-monitoring systems, as well as equipment to grow the brine shrimp and microscopic plankton the zebrafish eat.
Then Plavicki had to ship tanks of zebrafish from her postdoctoral lab in Wisconsin to Brown.
“It was challenging to move them here,” she said. “We had to bring the fish in the middle of winter. The fish did not like being shipped across the country in the cold. And then they did not want to breed. We had to wait until the next generation of fish to have good breeders.”
Even though zebrafish develop quickly — going from a fertilized egg to one-eighth-inch-long, transparent larva in three days — the best breeding fish are at least six months old.
At the same time Plavicki was establishing the breeding colony to produce the genetically engineered embryos and larvae needed for her research and procuring the right equipment to run her lab, she was also recruiting a team of junior researchers. Successfully bring aboard a group of talented, motivated scholars has been essential for establishing her innovative research, she said.

This summer, the Plavicki lab included three Brown undergraduates, three Ph.D. candidates from Brown’s pathobiology graduate program), two postdoctoral fellows and one research assistant (Rachel Koch).
“I am fortunate to have a really fabulous lab,” Plavicki said. “Having a good group of people is the foundation of all of our future work.”
Taking care of the colony
Even with the zebrafish colony established, keeping the lab running smoothly requires constant attention.
Every day, including weekends and holidays, the water quality — pH, conductivity and the nitrogen cycle — for both the central water system and a standalone quarantine water system needs to be checked twice daily to ensure the fish are in a healthy environment, Koch said. Also, the zebrafish are fed twice each day, and the brine shrimp and microscopic plankton for zebrafish food need to be grown and harvested daily.

Koch has primary responsibility for feeding the fish and checking on their living conditions; however, the lab employs a rotating schedule in which each member takes a turn at weekend care. The University’s animal care veterinarians assist with holiday care.
Koch also takes the lead on scheduling and ordering materials for the lab — from Petri dishes to dried brine shrimp eggs. She monitors the health of the fish and makes sure their tanks are clean. Like fish tanks everywhere, the zebrafish tanks are a favored habitat for algae. Algae are particularly fond of the upper tanks, closest to the fluorescent lights, she said.
When Koch transfers a shoal of zebrafish (fish of the same genetic line) from an algae-covered tank to a clean one, she scrubs off all the red algae and gives it a good rinse. Then she soaks the cleaned tanks in a bleach solution overnight to disinfect them. Next, she thoroughly rinses the tanks to get rid of all trace of bleach, which can harm the fish. Then the tanks air dry, at which point they are ready for new fish — either from another algae-covered tank or juvenile fish ready for a larger tank.
“I feed the fish every day and make sure everything is clean and all the water parameters are where they should be so the fish are in a good, healthy environment,” Koch said. “But it’s so much more than just working with fish. It’s been really interesting to learn about the genetics, and everyone in the lab is here when you need them.”
Studying contaminants that impact development
Though it takes a lot of work to care for a breeding colony of zebrafish, they are invaluable “lab rats” — model organisms — for biomedical research.

They are small, social animals with embryos that develop quickly outside of their mother and remain transparent for approximately a week, Plavicki said. It’s also much more cost-effective to maintain a colony of zebrafish than a colony of laboratory mice, another common model organism for research.
“Zebrafish are awesome,” Plavicki said. “They’re initially transparent so we can look under the microscope and watch them develop. We can look at blood flow. We can monitor cardiac function in an embryonic fish. We can do functional neuroimaging where we can look at changes in brain activity. Within the first week, all the major organs come on line. It’s very rapid.”
Using a high-speed scanning confocal microscope, Plavicki and her team can watch how exposure to toxicants or disrupted molecular pathways impact heart and brain development in real time. A confocal microscope uses lasers to illuminate tiny areas, which produces images and videos with much finer detail than is possible with a traditional light microscope.
Additionally, the zebrafish genome has been fully sequenced, and multiple genetic engineering tools have been developed for zebrafish research. Plavicki and her team use these tools to produce transgenic zebrafish lines that fluoresce green or red in specific cells — such as those destined to become heart muscle cells or specific neuron-support cells.
The ability to make certain cells glow — and more broadly, access to tools and technologies with similarly sophisticated capabilities — enable Plavicki and her team to explore distinctive questions about development and toxicants. One of the leading-edge topics the team researches is the effect of un- and under-studied PFASs on nervous system development and function. There are thousands of different PFAS contaminants, and only a few have been well-studied, Plavicki said.
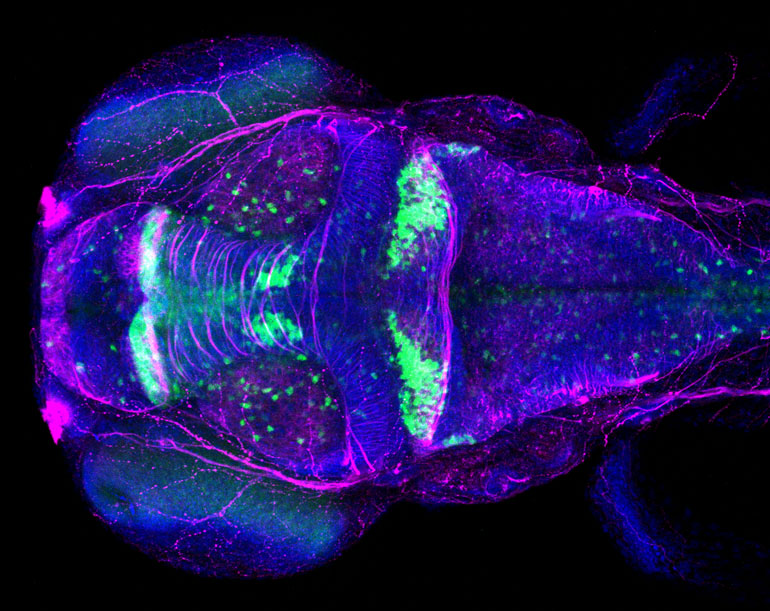
Even perfluorooctanoic acid (PFOA), one of the best-studied members of the group, has hosts of un-answered health questions. In fact, a recent scientific advisory committee led by a Brown professor questioned whether the current guidelines for PFAS water contamination levels were sufficient to protect human health.
“We don’t know which PFASs are really of health concern and which ones aren’t,” Plavicki said. “They are a hot topic in environmental science right now.”
Molecular signaling pathways
In addition to exposing zebrafish embryos to toxicants, the team also uses genetic tools to turn on and off the specific molecular signaling pathways hijacked by the toxicants, including a transcription factor involved in the development of blood cells, neurons and immune cells known as AHR.
Using an always-on version of AHR developed by one of Plavicki’s colleagues, Nathan Martin (the first graduate student to join the Plavicki lab) is investigating the role of AHR in the development of specific types of neurons and how exposure to contaminants that activate AHR alter brain development and function.

He is using confocal microscopy to look for large-scale changes in brain structure as well as brain activity when AHR is turned on in different types of neurons at different stages of development. Eventually, he will see if AHR activation impacts zebrafish behavior, such as their reaction to flashing lights or tapping sounds. These are common tests to determine if changes in brain development also impact perception and behaviors.
“Having the capability to see into something as it’s developing is so cool,” Martin said. “Since the zebrafish larvae are clear and since there are so many genetic lines that have been developed and the willingness to share resources in the zebrafish community, we can look at neuronal development. We can look at neuron function. We can look at vasculature development. We can look at the heart beat. It’s amazing.”
This work could lead to tweaking the blood-brain barrier, a highly selective border that separates circulating blood from the brain, to allow therapeutics to reach the brain.
Plavicki is also collaborating with Brown / Carney Institute for Brain Science neuroscientist Christopher Moore to manipulate genes in brain blood vessel cells using light.
“Both of our labs are interested in cerebral vascular development and function and issues surrounding the blood-brain barrier,” Plavicki said. “It’s been really exciting to pair our fish model with their mammalian models.”
In fact, it was the opportunity for just such collaborations — researchers working across fields of study in search of high-impact insights and solutions — that drew Plavicki to Brown and the Carney Institute after interviewing at a number of other research universities.
“Brown has this collaborative community of people where there really is cross-talk between departments,” Plavicki said. “I collaborate with labs in the department of neuroscience and with folks in pediatrics. It is a really creative, collaborative environment. That, in a nutshell, is the reason I chose to come to Brown.”