PROVIDENCE, R.I. [Brown University] — A team of researchers has found a new way to produce a polymer material called PBO, a product known commercially as Zylon that’s used in bulletproof vests and other high-performance fabrics. The new approach could be useful in making PBO products that resist degradation, a problem that has plagued PBO-based materials in the past.
“We show that using a nanoparticle catalyst, we can produce PBO in more environmentally friendly conditions and without using a chemical that’s known to cause these materials to degrade unexpectedly,” said Shouheng Sun, a professor of chemistry at Brown University and co-author of a new paper describing the research. “We think this could be a path toward making more robust PBO materials.”
The research is described in the journal Matter.
The traditional way to make PBO (its full name is polybenzoxazole) involves the use of polyphosphoric acid (PPA) as both a catalyst for necessary chemical reactions and as a solvent. PPA is a strong, highly corrosive acid and has been pinpointed as the source of PBO degradation. Molecules of the acid become lodged in the polymer chain, leaving the fibers susceptible to degradation when exposed to light and moisture over time. That degradation has led to the recall of PBO-based body armor in the past.
Sun’s lab at Brown has been working extensively with composite nanoparticle catalysts capable of performing the new reactions required to make PBO, and they do so without using PPA. Catalyzing the reactions with nanoparticles would also require less energy and can be performed using renewable formic acid as a hydrogen source. All of that makes the production process greener.
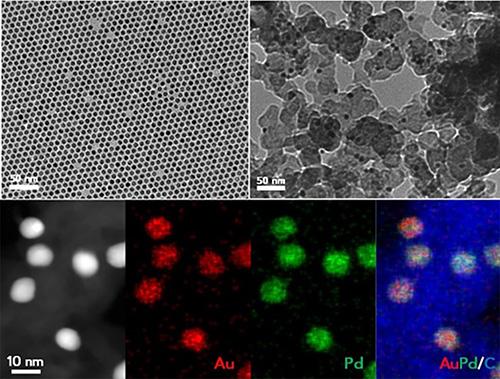
Up to now, however, composite nanoparticle catalysts have largely been used to make only small organic molecules. Whether a composite catalyst, which in this case is made from particles of gold and palladium alloys, could be used to catalyze the controlled growth of polymer chains was previously unknown.
“The key question we were trying to answer is if we can control the reactions so that we get a good control on the degree of polymerization,” Sun said. “We ultimately showed that we could do that by tuning the composition and size of the alloy nanoparticles in our catalyst.”
An alloy composition of close to 40 percent gold and 60 percent palladium was shown to be optimal for controlling the rate of reactions needed to make PBO. Particles around 8-nanometers in size produced a reaction speed that maximized the molecular weight of the PBO polymers.
To find out if the PBO was indeed resistant to degradation, the team worked with researchers in Brown’s School of Engineering to perform mechanical testing. Those tests showed that the PBO polymers made with the nanoparticle catalyst were more resistant to degradation than commercially available Zylon — even after being boiled in water and acid for days.
The researchers say that future work will focus on generating PBO polymers with higher molecular weights. The polymers generated for this study were significantly lighter than those of the commercial-grade product, which limits their initial mechanical strength. Still, the researchers say, the work is a strong proof-of-concept for the idea that composite nanoparticles can produce degradation-resistant PBO.
Jerome Robinson, an assistant professor of chemistry at Brown and a study co-author, noted that the diverse expertise of the Brown research team was critical to the success of this work.
“It was really important that we were able to collaborate with engineers and other researchers,” Robinson said. “To be able to walk across the street to the School of Engineering and do the mechanical testing was great, and I think we have the right team to carry this research forward.”
Other co-authors on the paper were Chao Yu, Xuefeng Guo, Zhouyang Yin, Zhonglong Zhao, Xing Li, Michelle Muzzio, Cintia Barbosa, Mengqi Shen, Yucheng Yuan, Junyu Wang, John Antolik, Gang Lu, Dong Su, Ou Chen, Pradeep Guduru and Christopher Seto. The early stage of the work was supported by the U.S. Army Research Laboratory and the U.S. Army Research Office (W911NF-15-1-0147). Additional support was provided by Brown’s Office of the Vice President for Research and the Institute of Molecular and Nanoscale Innovation.