PROVIDENCE, R.I. [Brown University] — For Ou Chen, working with artificial particles known as nanocrystals is like being in a wonderland. “These tiny particles can do so many amazing things,” said Chen, an assistant professor of chemistry, and they have comprised the core of his research since he came to Brown in 2015.
Chen and colleagues in his lab are currently in the process of making their nanocrystals part of state-of-the-art, window-like panels encasing a solar energy harvesting system. Around the world, roofs checkered with dark-blue solar panels have gained acceptance. These panels cover substantial space, but even a roof-full cannot gather enough energy to power a high-rise building. The panels Chen is designing are a potentially transformative solution.
A type of nanocrystal that his lab has been studying — the quantum dot — could be embedded in the windows to help capture and transfer light energy to standard solar cells lining the frame, which would then convert that energy to electricity. These solar panels would save space, as they would be easier to incorporate into buildings without obstructing daylight, so they could span entire skyscrapers and collectively generate ample electricity.
Nanocrystals hold promise in medicine as well, and Chen is aiming to maximize that potential through a collaboration with researchers at Rhode Island Hospital. Scientists can modify the particles so that, once injected into the bloodstream, they latch onto specific types of cells, such as cancer cells. Quantum dots’ abilities to absorb and emit light would allow medical professionals to image targeted cells and detect their locations. Quantum dots can also efficiently use electricity to release colored light — a property that makes them apt alternatives for components of television, computer, smartphone and tablet displays.
Chen said nanocrystals’ adaptability to a variety of applications is due in large part to their countless conceivable combinations. Each nanocrystal is a highly organized arrangement of atoms, so that synthesizing them from different elements and attaching chemical molecules called “ligands” to the particles’ surface yields their unique properties.
Chen’s accomplishments and prospects for his research have already garnered significant attention. In 2020 alone, he earned numerous accolades: an early career fellowship from the Sloan Foundation, an Early Career Development (CAREER) award from the National Science Foundation, and a three-year 3M Non-Tenured Faculty Award. He was also named a Camille Dreyfus Teacher-Scholar.
Chen has spent plenty of time working as a chemist, but he simultaneously takes on the role of an engineer, creating and piecing together particles about a millionth of a millimeter in size.
When he analyzes the resulting structures with an electron microscope, a kaleidoscope of black-and-white shapes emerges. Within these images lie collections of nanocrystals that are visual evidence of the significant advances Chen’s lab has achieved, and he tests how the particles interact with light, electricity, magnetism and other physical phenomena.
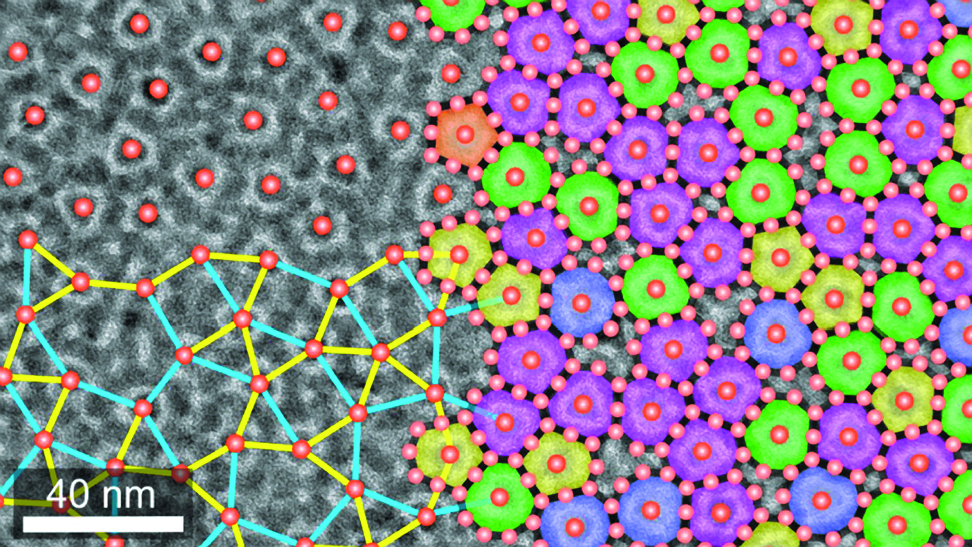
Chen assembles these chemical “building blocks” into macroscale superstructures and creates cutting-edge technology. But much about these superstructures and nanocrystals is not yet clear: “The underlying mechanism is still not fully understood,” Chen said.
His expertise in inorganic chemistry has enabled his lab to experiment with the chemical composition of individual nanocrystals. His lab recently developed a quantum dot unique in its tetrahedral shape, which resembles a triangular pyramid and allows for the formation of sturdier, more complex materials. This breakthrough not only earned publication in the journal Nature in 2018, but also instigated another fruitful investigation, featured in the journal Science.
Chen’s latest discovery of a single-component quasicrystal came about from his study of stacking nanocrystals on top of a solution as opposed to on a solid surface. In the course of this experimenting, Chen and his team observed an unexpected capability of their quantum dots: formation of a quasicrystal in solution.
Quasicrystals are ordered structures without a repeated pattern that can be used to make unique materials, like non-stick cooking pans, but they typically consist of two different kinds of components, such as two types of atoms. Chen’s lab became the first to show a one-component quasicrystal, whose applications remain to be explored but may enable researchers to convert standard materials into novel ones, such as by making metals transparent or magnets that can shape-shift.
Chen believes the remarkable chemical and physical properties of nanocrystals can be harnessed to advance technology in many more ways. “We can create very, very high-quality, precisely controlled nanocrystals,” he said. “Then we can use that for further discovery.”
Brown Chemistry Department Chair Lai-Sheng Wang said of Chen’s work, “He can turn ordinary materials into something wonderful. He came here and just made an immediate impact.”
“We are doing a lot of fundamental research to keep pushing the boundary moving forward,” Chen said, “but my big dream is that we can really create materials for applications that have the possibility to change people’s lives.”
