Brown professor develops AI system to detect danger in blood vessels
The lab of George Karniadakis, professor of applied mathematics and engineering, leads the charge of developing physics-informed neural networks to diagnose and predict the severity of arterial aneurysms.
PROVIDENCE, R.I. [Brown University] — Throughout his research career, Professor of Applied Mathematics and Engineering George Karniadakis has pioneered diverse computational methods for multi-scale problems in science and engineering. More recently, riding the artificial intelligence (AI) wave of the last decade, his team has introduced the use of physics-informed neural networks to diagnose the severity of arterial aneurysms.
Aneurysms are bulges in the arteries caused by blood pressure pushing a weakened section of the arterial wall outward. Over time, arterial blood flow can lead to increased pressure on these sack-like structures, leading to their growth, weakening and eventual rupture.
Karniadakis’s lab uses computational biology to model biological processes. His work on aneurysms began 15 years ago when he partnered with Joseph Madsen, a pediatric neurosurgeon at Boston Children’s Hospital, and the late Peter Richardson, a professor of engineering at Brown.
Although aneurysms can occur anywhere in the body, one of the most common locations is the brain. Rupture can cause massive stroke and nearly half the time leads to death, according to the Brain Aneurysm Foundation. However, brain surgery to remove the aneurysm is inherently risky and can itself result in brain damage or stroke. For this reason, physicians only operate on high-risk aneurysms.
“ We wanted to replace a subjective, qualitative assessment done by different neurosurgeons with something very objective and quantitative. ”
A major issue neurosurgeons face is determining if and when to operate, Karniadakis said. Using X-ray and MRI techniques, “doctors simply look at the aneurysm with the naked eye and say, ‘Oh, that doesn’t look good, I better operate,'” he explained. “It’s totally qualitative. We wanted to replace a subjective, qualitative assessment done by different neurosurgeons with something very objective and quantitative.”
The researchers focused on the circle of Willis, a ring of blood vessels connecting the four major arteries of the brain that is particularly susceptible to aneurysm formation. They devised a way to use neural networks, which are a type of AI that consists of thousands of processing nodes designed to mimic neurons in the human brain, informed by mathematical equations that describe blood flow to predict aneurysm rupture.
Using visual data from MRI scans to construct the geometry of the aneurysm, and video of flow visualization with contrast agent that the doctor injects into the patient, the neural networks computed blood flow and pressure and inserted these values into a physics equation governing fluid dynamics. If the equation result didn’t match these calculations, the “neurons” were able to “learn” and modify their result until they determined correct flow and pressure values. The researchers termed this system physics-informed neural networks.
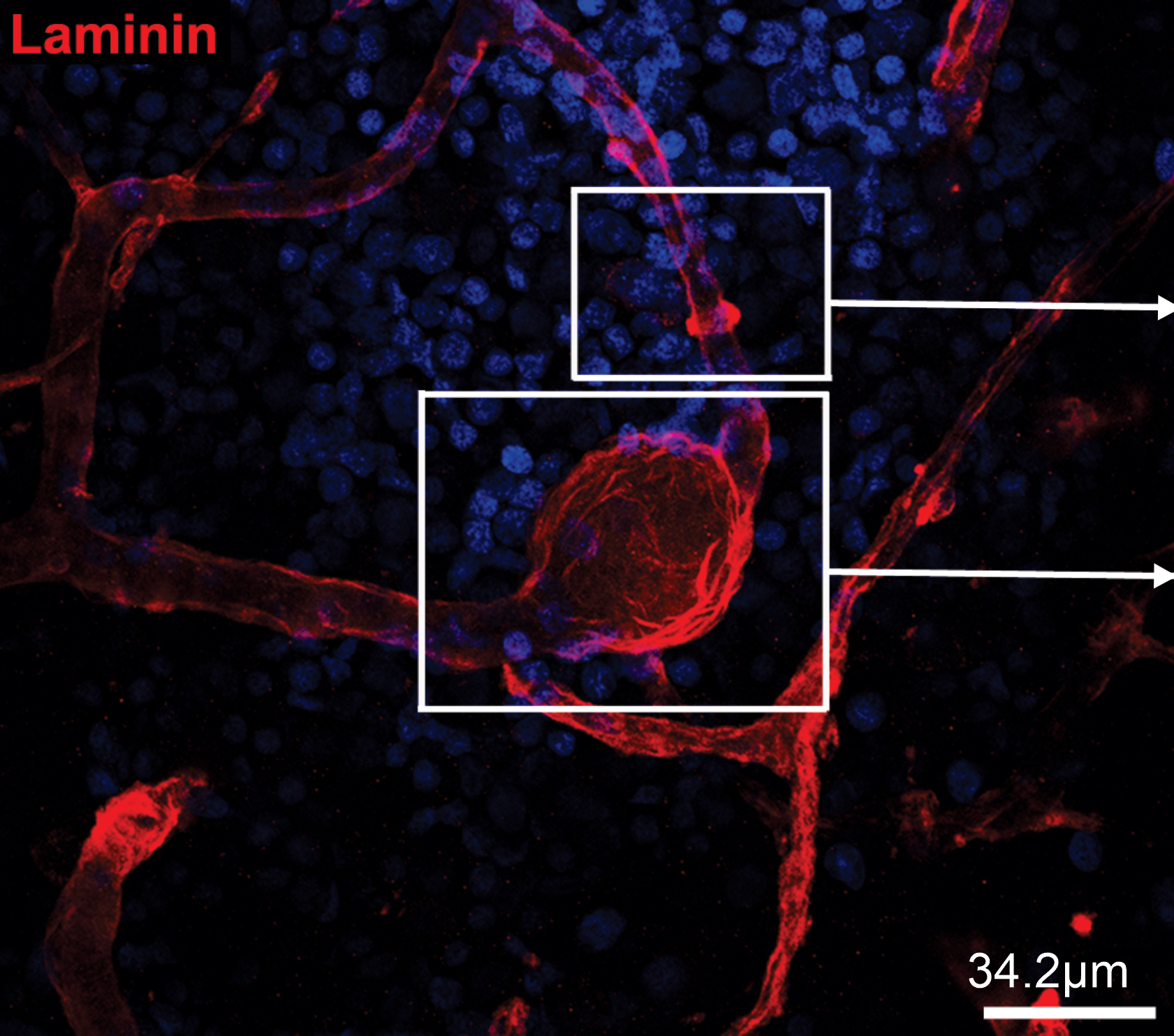
“Physics-informed neural networks (PINNs) are a way to bring physics and mathematics into the neural network,” Karniadakis said. “We can zoom in on the aneurysmal sac where the problem is…and predict when the rupture will happen based on objective criteria.”
Karniadakis’s lab has recently collaborated with researchers from the Massachusetts Institute of Technology and Nanyang Technological University in Singapore, using PINNs to create an AI system that can identify and measure blood flow within retinal microaneurysms for diabetic retinopathy (DR). Karniadakis and his main Brown collaborator He Li, who is assistant professor of engineering at Brown’s Center for Biomedical Engineering, also collaborate with Jennifer Sun and Konstantina Sampani from Harvard’s Joslin Diabetes Center.
“The vessels are very small, but if the (aneurysms) dilate and then rupture, the patient will be blinded,” Karniadakis said.
These microaneurysms — too small to be detected with the naked eye — occur in patients with diabetic retinopathy when uncontrolled blood sugars damage retinal blood vessels in the back of the eye. Because of their minuscule size, physicians face the additional challenge of detecting the microaneurysms before they can treat them.
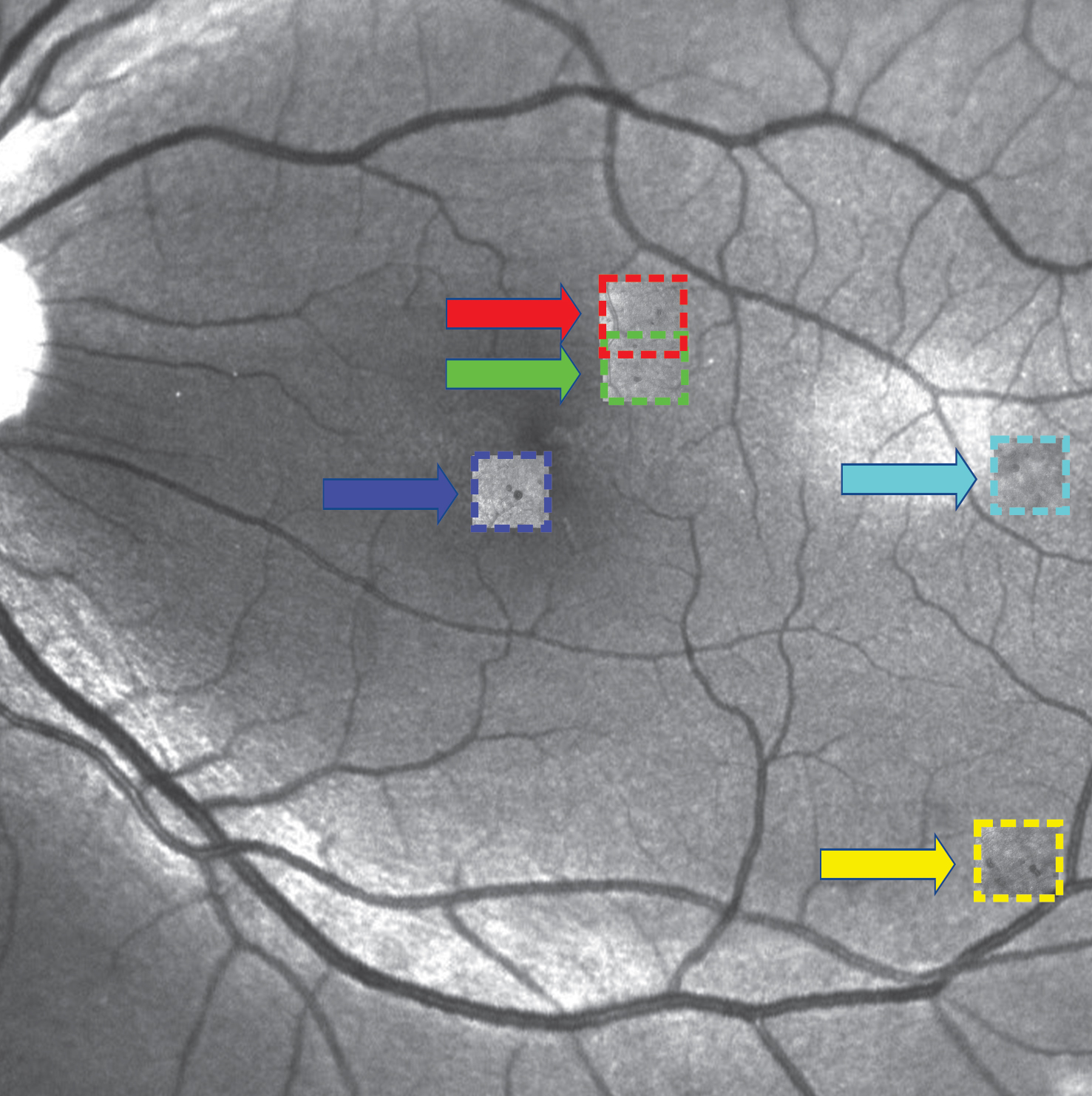
“We’re developing other neural networks to find the aneurysms and tell us their shapes, sizes, and what they look like,” Karniadakis said.
The researchers created “microaneurysms-on-a-chip,” microfluidic devices with bulges of different shapes and sizes that mimic microaneurysms. By practicing the analysis of fluid flow through these devices, the PINNs were trained to calculate the velocity of blood in real patient microaneurysms — valuable information physicians can use to judge risk of rupture. The results of this artificial intelligence velocimetry, or AIV, were published in the Proceedings of the National Academy of Sciences.
“We are trying to make this a product,” Karniadakis said, adding that the researchers have been working with a company to get these PINNs on the market so that they can benefit patients suffering from aneurysms. Before they can do that, however, the product must go through clinical trials and then FDA approval.
Currently, Karniadakis and his team are working on developing new AI to identify these microaneurysms among what he calls the “forest of blood vessels” and classify their severity based on shape. They have developed the AOSLO-net, which can automatically identify and segment microaneurysms from images acquired with adaptive optics scanning laser ophthalmoscopy in Sun’s lab at the Joslin Diabetes Center.
“Ultimately, the aim of the Brown-Harvard team is to develop a new integrated imaging and modeling framework using advanced AI models (such as AIV and AOSLO-net) that could facilitate the pathological study of diabetic retinopathy and help ophthalmologists make disease prognoses,” Karniadakis said.
Success would help shift the paradigm from conventional DR screening approaches based on fundus images — pictures of the back of the eye taken with retinal cameras — to more advanced imaging modalities.
“This framework can potentially be extended to investigate critical hemodynamic markers for other cardio- and cerebrovascular diseases,” Karniadakis said.
