PROVIDENCE, R.I. [Brown University] — A team of researchers from Brown University has developed a new nanotechnology-based approach that could improve treatment of fungal infections, particularly those caused by the increasingly drug-resistant Candida species.
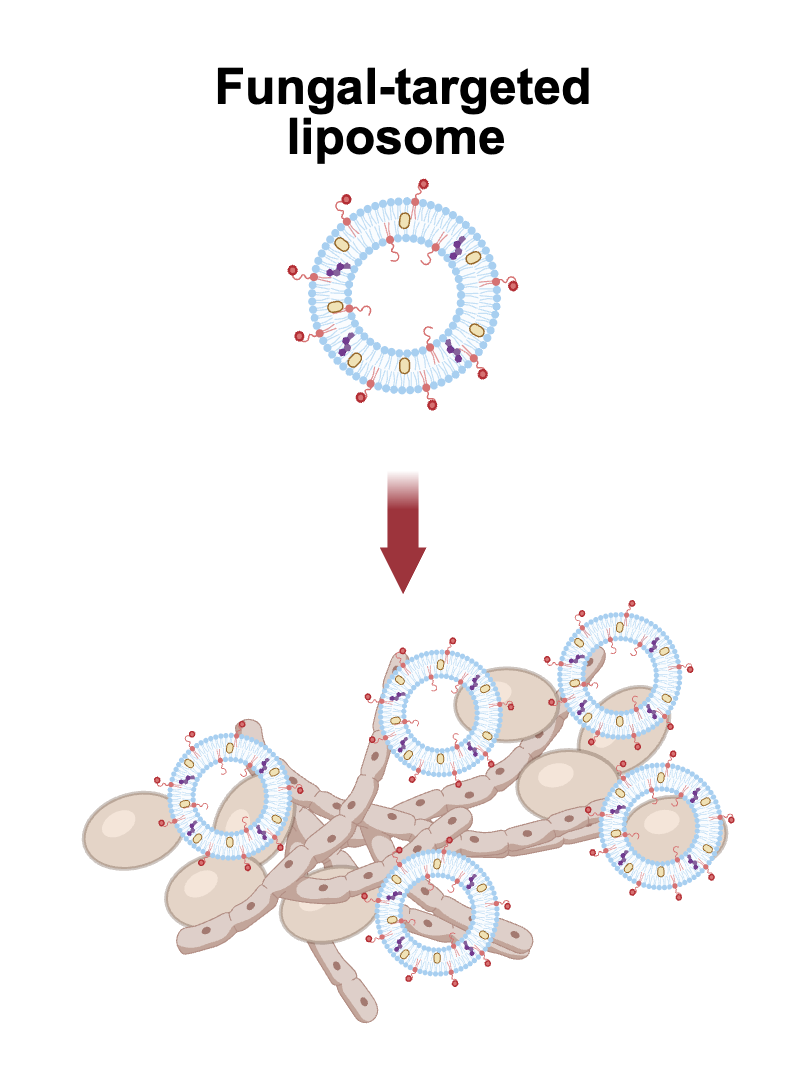
In a new study, the team demonstrated a method for targeting liposomes — tiny lipid-based nanoparticle drug delivery systems — directly to fungal cells. The study found that the new approach dramatically increased drug effectiveness, even against tough-to-treat fungal biofilms, without harming healthy human cells.
“Fungal infections can be extremely difficult to treat and the drugs at doctors’ disposal are limited,” said Veronica LaMastro, a recent Ph.D. graduate in biomedical engineering at Brown and the study’s lead author. “By adding a targeting peptide on the surface of liposomes, we can better target the delivery of an anti-fungal drug to fungal cells, and increase its ability to kill those cells.”
The research, which is supported by the National Science Foundation, is published in the journal Advanced Functional Materials.
Candida species are common fungi that live harmlessly in and on the human body. But for people with weakened immune systems, including cancer patients, transplant recipients or those in intensive care, Candida can turn deadly. C. auris, in particular, has emerged as a “superbug,” spreading rapidly through health care systems and resisting many frontline drugs. Between 2017 and 2018, C. auris infections increased more than 300% in the United States alone.
To tackle this problem, the Brown team turned to liposomes, spherical nanoparticle packages made from natural and synthetic fats. These structures can carry drugs inside their hollow centers or within their fatty membranes, and can be used to improve the delivery and stability of drug therapies. The major advance in this new approach was to “decorate” the outer surface of the liposomes with a peptide — a short chain of amino acids — that is naturally attracted to Candida cells. The peptides act as a molecular homing device, helping the liposomes recognize and bind to Candida cells, LaMastro explained.
After screening several targeting peptides, the researchers found that one called penetratin was most effective in targeting Candida. The team then synthesized liposomes decorated with penetratin and containing an FDA-approved antifungal drug called posaconazole, which is currently used as a prophylactic agent to prevent Candida overgrowth.Once they had developed their targeted liposomes, the team put them to the test in a series of lab experiments.
Lab tests showed that liposomes decorated with penetratin were significantly more likely to interact with Candida cells than standard liposomes, confirming the effectiveness of the targeting strategy. The targeted delivery system also dramatically increased the potency of the antifungal drug. It inhibited Candida growth at concentrations up to eight times lower than those required for free posaconazole and prevented biofilm formation at doses up to 1,300 times lower, the researchers found. The approach also appears to be generally safe: The targeted liposomes showed no toxicity to human cells commonly affected during infection, including cells found in skin, blood vessels, vaginal tissue and red blood cells, according to the study.
To test how well the treatment might work in a real-world infection, the team used a mouse model of intradermal C. albicans infection. Mice that received the targeted liposomes had a 60% lower fungal burden than those given regular drug-loaded liposomes, suggesting a real benefit in preventing fungal spread.
Taken together, the research suggests that targeted liposomes are a promising new method for fighting fungal infections, which are of substantial clinical importance.
“Fungal infections are a vastly understudied area, especially in the engineering and biomaterials communities,” said Anita Shukla, a professor in Brown’s School of Engineering who directed the research in her lab. “But with rising antimicrobial resistance coupled to the increasing use of antifungals in clinical and agricultural settings, this type of work becomes more important. We hope more researchers will recognize that and do more work in this field.”
The team plans to continue studying and expanding their method, Shukla said. This study looked at a drug that is generally used to prevent Candida infections. The team now plans to test it with drugs used to treat already-established infections.
The research was supported by the National Science Foundation (CBET-1942418). Additional co-authors were Dominique Walker, Joanne Liu and Tobias Meng-Saccoccio.