PROVIDENCE, R.I. [Brown University] — A new $25 million gift to Brown University will fuel scientific discovery and help innovative new ideas in brain science get off the ground.
The generous gift from a donor who wishes to remain anonymous will support research in computational brain science, enabling Brown to attract and retain world-class teachers and scholars, and it will endow an innovation awards program at the University’s Robert J. and Nancy D. Carney Institute for Brain Science to provide seed funding for new high-impact research in computation and other areas of brain science.
“This transformational gift recognizes the momentum that continues to build at Brown University, where our talented faculty are leading scientific discovery including in the rapidly emerging specialty of computational brain science,” said Diane Lipscombe, director of the Carney Institute and a professor of neuroscience. “This gift will allow us to sustain a culture of innovation, which has led to an impressive number of discoveries and returned countless new grants to Brown that forge new areas of research.”
With more than 180 affiliated faculty members in 20 units and eight affiliated graduate programs, the Carney Institute is pursuing research that has real-life, human applications, Lipscombe said. Core areas of research include work on innovative advances in computational brain science to investigate cognition, behavior and mood disorders; novel technologies to interface with the brain to understand brain circuits and restore lost functions; and research into the mechanisms of cell death to identify therapies for neurodegeneration, such as in amyotrophic lateral sclerosis (ALS) and Parkinson’s and Alzheimer’s diseases.
This new $25 million gift is part of the University’s $3-billion BrownTogether campaign, which has raised $2.74 billion to date. It also builds upon significant philanthropic investment in Brown’s cutting-edge work in brain science — of the total contributed by donors to date, more than $187 million has been raised to support research and education in brain science, including a $100 million gift that named the institute in 2018. The gifts support a core research priority in Brown’s Building on Distinction strategic plan: “understanding the human brain.”
Launching bold ideas
In 2014, the Carney Institute launched an innovation awards program to support early-stage research projects that are too new to attract external funding but have great potential to advance science and benefit society. The new gift will endow a similar innovation awards program, ensuring that the Carney Institute will be able to sustainably invest in innovation for years to come.
Ashley Webb, an assistant professor of molecular biology, cell biology and biochemistry at Brown, received an innovation award in 2019 to establish a new system to study how neurons age. She is developing a cell reprogramming platform, called “direct reprogramming,” to study aging in the hypothalamus brain region. The hypothalamus controls critical processes, such as sleep, temperature regulation, eating and metabolism, which can become dysregulated with aging. With direct reprogramming, Webb is able to convert a skin cell, for example, into a brain cell that maintains the hallmarks of aging.
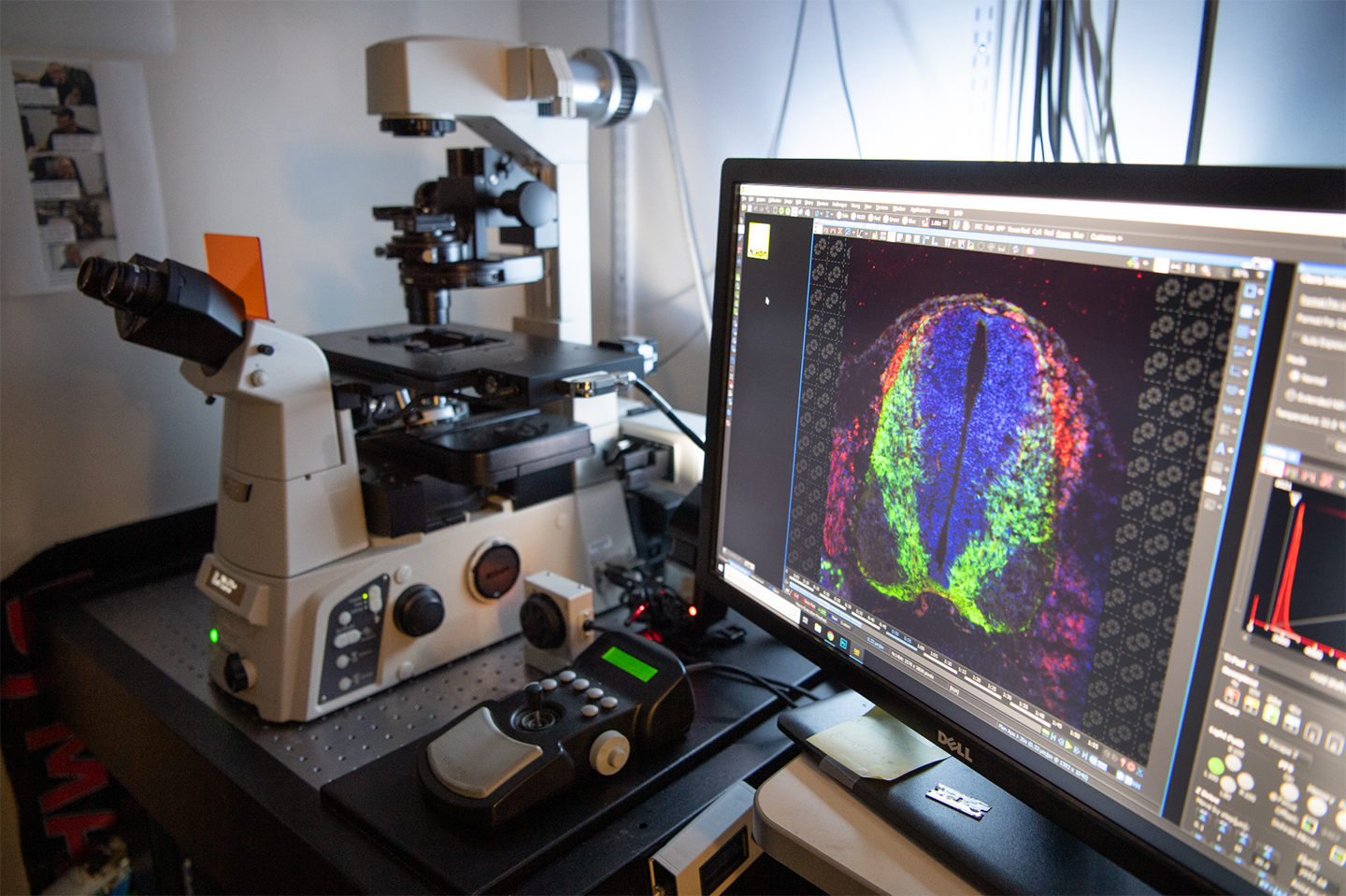
Starting from mouse skin cells, Webb’s group generated rare hypothalamic brain cells, called POMC neurons, that are important for metabolic health and weight control. Normally, these cells trigger satiety (the feeling of being full), but they lose the ability to do so as they age.
Webb is now applying the same approach to create POMC neurons from human cells, which for the first time will allow researchers to generate rare types of human neurons that are physiologically aged.
“If we take cells from aged individuals, the neurons we generate actually retain damage associated with aging,” Webb said. “This allows us to compare young and aged neurons, and understand why the old neurons don’t function as well. We believe this platform will give us important insight into why metabolism is altered with age and in many diseases.”
The Innovation Awards Program, Webb said, gave her group the freedom to take on a bold, early-stage idea. Her team hopes to use the direct reprogramming approach to generate neurons from individuals with brain diseases and disorders, such as Alzheimer’s.

