Researchers identify neuronal populations important for odor memories

Odors have long been used by animals as cues to navigate their environment, but little is known about the neural mechanisms behind the creation of odor memories. Using genetic tagging, a recent study co-authored by Alexander Fleischmann, a neuroscientist at Brown University, identifies specific populations of neurons in the olfactory cortex that are activated in this process.
Published last month in the journal Current Biology, the study sought to understand, at a cellular circuit level, “how odor memories are generated and (determine) the mechanisms that allow the brain to store and retrieve odor context, which is shaped by experience,” said Fleischmann, an associate professor of neuroscience who is affiliated with Brown’s Carney Institute for Brain Science.
The identification of these neural populations may be the link between the olfactory cortex with brain regions associated with higher-order functions like learning and memory.
The researchers fear-conditioned mice to associate the smell of ethyl acetate—an odor that is relatively neutral and doesn’t induce aversive behavior by itself—with a foot shock. Three days following the training, the researchers tested the mice to determine if they had learned the association.
Researchers measured the “escape behavior” of the mice, including the distance they ran from the odor and their increase in speed. Genetic tagging then allowed researchers to “visualize neurons (that) were activated” during learning, according to Fleischmann.
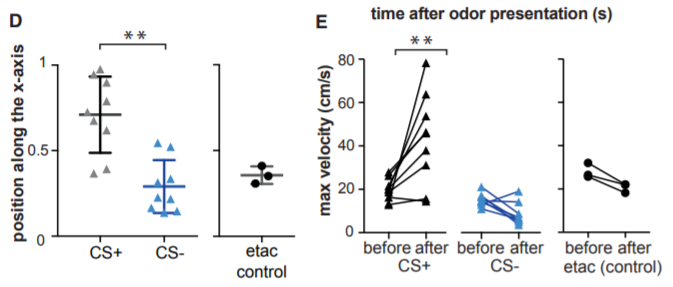
Figure E: The mice moved faster after presentation of the odor in the CS+ condition compared to the CS- and etac control conditions.
In the next part of the experiment, the researchers found that when these populations of neurons were chemogenetically silenced, the mice did not run as far or as fast. This suggests that the mice only retained a partial memory of their learning and supports the notion that these neural populations play an important role in odor memory, according to the paper.
The researchers then performed the opposite modification on a different group of mice – rather than silencing these neuron populations, they chemogenetically reactivated them. Tested in an open field, the mice explored less when they smelled ethyl acetate compared to a control group whose neurons had not been reactivated. These results suggest that reactivation contributes to the mice’s recall of prior learning, Fleischmann said.
As a follow-up on this study, Fleischmann and researchers in his lab are currently investigating why these specific neuron populations play a role in memory, using imaging and other molecular genetics techniques. Some of the questions they hope to answer, Fleischmann said, are what these neurons respond to, where they transmit information to, and how they are able to learn and communicate the context of the odor.
Fleischmann’s research on odor memory may also contribute to society’s understanding of Alzheimer’s disease. “The olfactory system is amongst the first (brain functions) that deteriorate when people develop Alzheimer’s disease symptoms,” Fleischmann said. “In the clinic, people are screened for olfactory memory deficits when they go through Alzheimer’s testing.”
Fleischmann said he hopes to “use mouse models for Alzheimer’s disease to see how olfactory memory traces are affected in these model systems.”
Other co-authors of the paper include Claire Meissner-Bernard, Yulia Dembitskaya, and Laurent Venance of the Center for Interdisciplinary Research in Biology at the Collège de France. This research was funded by the Amorçage de Jeunes Équipes program of the Fondation pour la Recherche Médicale and a postdoctoral fellowship by LabEx MemoLife.



