Carney Institute grants $564,000 in innovation awards to Brown scientists

Through an innovation awards program, the Robert J. and Nancy D. Carney Institute for Brain Science will provide $564,000 in seed funding for new high-impact research in computation, visual science, Parkinson’s disease and Alzheimer’s disease.
In 2014, the Carney Institute launched the Zimmerman Innovation Awards in Brain Science to support early-stage research projects that are too risky and new to attract external funding but have great potential to advance science and benefit society. All areas of brain science research are considered and all Brown faculty members conducting brain science research at Brown University or its affiliated hospitals are eligible. This year, the institute will support six collaborative projects led by 10 Brown University scientists, bringing the number of innovative brain science research projects supported since the program’s inception to 38.
“This year’s research awards represent the spirit of innovation on many levels,” said Diane Lipscombe, Reliance Dhirubhai Ambani Director of the Carney Institute. “The researchers are exploring the capacity of imagination, and the factors driving social behavior. They are applying machine learning to develop patient-specific treatments for Parkinson’s disease, and engineering a device to restore vision. They are tackling Alzheimer’s disease in unique ways, addressing gender equity in health-related research, and developing approaches for very early identification of people at risk for Alzheimer's disease while still in their teens. Great ideas have to be tested, so we are thrilled to help launch these exciting early-stage research projects.”
The Carney Institute invests up to $100,000 per project for one year, renewable for a second year on a competitive basis. In addition, a pool of funds is available to specifically support projects addressing Alzheimer’s disease and related dementias. This year, Carney was able to award $232,000 to projects addressing Alzheimer’s disease.
Below are the projects funded by this year's innovation awards.
aiDBS: artificial intelligence in deep brain stimulation for treatment of Parkinson's disease
Investigators: Shane Lee, assistant professor of neurosurgery, and Wael Asaad, Sidney A. Fox and Dorothea Doctors Fox Associate Professor of Ophthalmology and Visual Science, associate professor of neurosurgery and associate professor of neuroscience
Although people with Parkinson’s present many different symptoms rapidly, the current state-of-the-art neuromodulatory therapy for the disease, closed-loop deep brain stimulation (closed-loop DBS), works on a slower timescale and is not optimized to treat the whole constellation of exhibited symptoms. By adding artificial intelligence models to the existing closed-loop technology, Lee and Asaad seek to first predict and then address multiple symptoms of Parkinson’s all at once and on a rapid timescale. If successful, their study has the potential to greatly impact the field of closed-loop DBS, the researchers said.
A multi-areal wireless cortical implant for closed-loop visual prosthesis
Investigator: Farah Laiwalla, assistant professor of engineering
Drawing on years of neuroprosthetics research, Laiwalla will create and deploy up to six cortical implant systems designed to restore patients' vision. These devices will implement a never-before-used combination of distributed wireless implant architecture, sub-millimeter sized microimplants and bidirectional neural interfacing. "Our novel technology has the potential to enable access to visual processing in an unprecedented way, and is anticipated to transform how we address profound visual impairment," said Laiwalla.
Imagining the future: computational models and brain mechanisms of mental simulation
Investigators: Thomas Serre, Associate Director of the Center for Computational Brain Science, Director for the Center for Computation and Visualization, professor of cognitive, linguistic and psychological sciences and professor of computer science, and David Sheinberg, professor of neuroscience

will be taking the lead on the behavioral and neurophysiological
aspects of the project.
How do we use imagination — our ability to create experiences of the world in our own minds — to solve real-world problems? Is this capacity uniquely human? And how does it differ from actual experience? Sheinberg and Serre will study these questions at the level of individual neurons and computational models. They will train both animals and artificial neural networks to solve a complex visual task that relies on mental simulation. Their goal is to understand how specific neural architectures support mental simulation and how people and machines can use these imagined experiences to guide decisions.
Visualizing sites of neuromodulation in mice
Investigator: Gilad Barnea, Sidney A. Fox and Dorothea Doctors Fox Professor of Ophthalmology, Visual Science and Neuroscience, Director of the Center for the Neurobiology of Cells and Circuits
As a part of the wider mission to understand how animals’ internal states and experiences shape sensory processing to produce behavior, Barnea and his team will develop what he refers to as “a hypothesis-generating technique” which enables selective, brain-wide examination of sites of neuromodulation with cellular resolution in mice. Using the neuropeptides oxytocin and vasopressin as proof of concept, Barnea’s analysis will allow scientists to assess the roles played by these modulatory systems in social behavior. Since the oxytocin receptor and one of the vasopressin receptors are involved in social behavior and have a strong risk score for autism spectrum disorder, one immediate outcome of Barnea’s work is researchers’ ability to examine the role these neuropeptides play in mouse models of autism spectrum disorder with much higher resolution and precision than currently possible.
PROJECTS TACKLING ALZHEIMER’S DISEASE AND RELATED DEMENTIAS
Defining the contribution of ovaries to the onset and progression of Alzheimer’s disease
Investigators: Richard Freiman, professor of molecular biology, cell biology and biochemistry, professor of obstetrics and gynecology, and Gregorio Valdez, GLF Translational Associate Professor of Molecular Biology, Cell Biology and Biochemistry (MCB) and Co-Director of the MCB Graduate Program
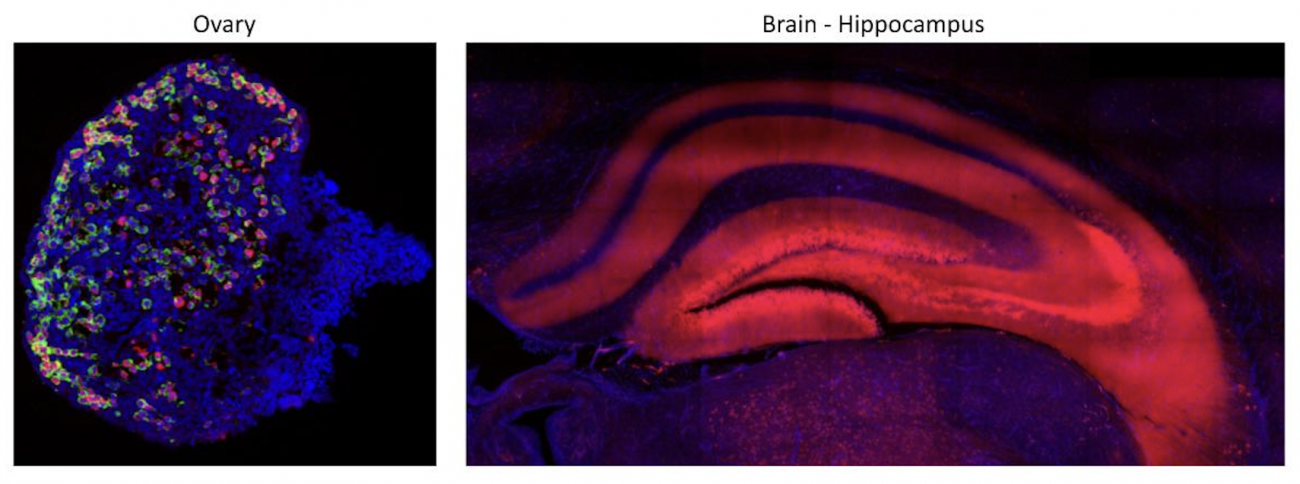
and mouse hippocampus (right)
Women are twice as likely to develop Alzheimer’s disease as men. Yet, there is a dearth of information about the relationship between female sex characteristics and the onset and progression of the disease. Freiman and Valdez will run an integrative series of pilot studies with mice to define whether changes in the ovary contribute to the onset and progression of Alzheimer's disease. One group of experiments will examine the ovaries prior to, at the onset and during the progression of pathology in diverse mouse models of Alzheimer’s; the other group will examine the onset and severity of Alzheimer’s in mice with and without healthy ovarian functions. By studying the integration of the brain and ovaries, their project will not only contribute to potential therapeutic opportunities that prevent or slow a devastating neurodegenerative disease in women, but it will also address the imminent need to improve health-related research inequities between women and men.
Sleep in adolescents at genetic risk for Alzheimer's Disease: a missing link to early life detection
Investigators: Jared Saletin, assistant professor of psychiatry and human behavior, and Mary Carskadon, professor of psychiatry and human behavior

E.P. Bradley Hospital Sleep Research Laboratory
Saletin and Carskadon seek to test an intriguing new possibility: whether alterations in brain and behavioral sleep-related mechanistic markers are already present early in life in adolescents at genetic risk for Alzheimer’s disease. Although adolescence is too early to express symptoms or visible plaques, early life consequences of the gene APOE4 have been observed in sleep-related phenotypes including hippocampus structure, memory and IQ. Thus, early changes in sleep — and potentially in brain makers of sleep-related glymphatic flow — may occur in at-risk youth. If findings in Saletin and Carskadon’s pilot project support this premise, they will open the door for early sleep-focused intervention long before Alzheimer’s symptoms emerge.



