Carney Institute grants more than $600,000 in innovation awards to Brown University scientists

This year’s projects span behavioral and systems neuroscience, circuit therapeutics, computation and modeling, molecular analysis, and Alzheimer’s disease research.
Through an innovation awards program, the Robert J. and Nancy D. Carney Institute for Brain Science will provide $614,000 in seed funding for new, high-impact research in brain science, including $250,000 for projects focused on Alzheimer’s disease and related dementias. The funding will support the work of Carney-affiliated researchers to break new ground in projects that span computation and modeling, molecular analysis, behavioral and systems neuroscience, and circuit therapeutics.
A decade ago, the Carney Institute launched the Zimmerman Innovation Awards in Brain Science to support early-stage research projects that are too risky and new to attract external funding but have great potential to advance science and benefit society. This year’s cycle of six awards, including three spanning broadly across brain science and three focused on Alzheimer’s disease and related dementias, brings the total amount the program has granted to innovative research since its inception to nearly $5,000,000.
“Collaboration accelerates advances in brain science and this year’s awards support some outstanding and unique collaborations, from basic mechanisms to clinical application,” said Diane Lipscombe, Reliance Dhirubhai Ambani Director of the Carney Institute. “Researchers have teamed up to tackle high-impact questions: How do neurons make the right connections during early nervous system development? Can focused ultrasound be used as a neural circuit therapy for neuropsychiatric disorders? Can understanding the neural circuits that give rise to visual imagination create new approaches to treat conditions like PTSD?”
“Similarly, brain scientists are exploring the frontiers of knowledge to change the trajectory of Alzheimer's disease with three innovative projects: two directed at early-stage drug targeting of pathology associated with Alzheimer's disease, and one aimed at developing an early-stage blood-based biomarker for neurodegeneration. Such research is essential to accelerate the development of new treatments and early detection for Alzheimer's disease and related dementias.”
The Carney Institute invests up to $100,000 per project for one year, renewable for a second year on a competitive basis. Projects led by a junior faculty member are eligible for an additional $32,000 in funding.
Below are the projects funded by this year's innovation awards.
Axon guidance via changes in growth cone membrane potential
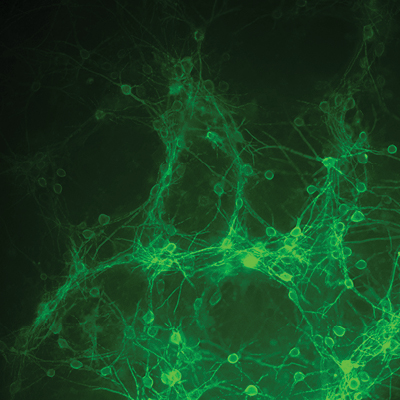
Abdelfattah Lab’s new voltage indicators, enabling the Jaworski Lab
to measure the membrane potential at the tip of axons.
Investigators: Alexander Jaworski, June G. Zimmerman Associate Professor of Brain Science, and Ahmed Abdelfattah, Robert J. and Nancy D. Carney University Assistant Professor of Brain Science
The Jaworski and Abdelfattah labs will put their cutting-edge molecular and biophysical tools together to answer questions about how neurons form precise connections with each other during embryonic development–information important to understanding causes of neural circuit dysfunction and disease. To make connections, neurons send out slender fibers called axons which find their targets with remarkable accuracy, a process called axon pathfinding. The Abdelfattah Lab is developing genetically encoded, highly sensitive voltage indicators that enable researchers to measure small changes in electrical potential across cell membranes. From there, the Jaworski Lab will use these indicators to observe a structure at the tip of the axon called the growth cone. By measuring changes in the membrane potential of the growth cone during axon pathfinding, the team will get unprecedented insight into how axons respond to the signals that guide them to their targets. “This work promises to have a major impact in the field of axon pathfinding by unraveling fundamental mechanisms of growth cone steering. Such knowledge has the potential to inform therapeutic approaches for neural circuit repair in various disease settings,” said the team.
Development of low intensity focused ultrasound protocols for selective modulation of cortico-thalamic neurons relevant to neuropsychiatric disease
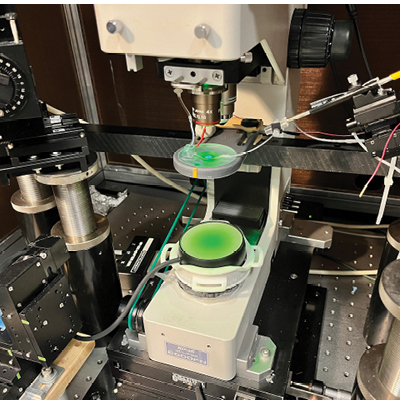
fluorescence imaging of voltage responses in in vitro neurons
during low intensity focused ultrasound stimulation.
Investigators: Brian Theyel, assistant professor of psychiatry and human behavior, Frederic Pouille, assistant professor of neuroscience (research), and Linda Carpenter, professor of psychiatry and human behavior
Theyel, Pouille and Carpenter will work together to investigate low intensity focused ultrasound stimulation–an emerging neuromodulation technology–as a safe, non-invasive potential treatment for schizophrenia and autism spectrum disorder. Using Carpenter’s ultrasound equipment, the Theyel and Pouille labs will attempt to alter two sensory circuits that researchers suspect contribute to these disorders: the feedforward and feedback cortico-thalamo-cortical pathways. Using Voltron, a fluorescent sensor imaging technology with unprecedented ability to visualize neurons developed at Brown University by the Abdelfattah Lab, Theyel and Pouille will test their hypothesis by imaging the activity of cortico-thalamic neurons during low intensity focused ultrasound stimulation. “It is our hope that our findings will yield tractable results for the strong neuromodulation community at Brown University to use in clinical studies of schizophrenia and autism spectrum disorder,” said the team.
“(Re) Imagining the future: computational models and brain mechanisms of mental simulation.”
Investigators: Thomas Serre, Associate Director of the Center for Computational Brain Science, Director for the Center for Computation and Visualization, professor of cognitive, linguistic and psychological sciences and professor of computer science, and David Sheinberg, professor of neuroscience
The Serre and Sheinberg labs are in the second year of their project investigating mental simulation, or how we build a visual picture in our minds. Thanks to ultra high-channel count probes enabled by their first year of funding, the team is currently able to study real-time neural activity in animals with much greater detail than fMRI imaging can provide. Through Sheinberg’s data and Serre’s ability to decode it, the team will use their renewal for a second year of funding to begin to understand the circuitry involved in the complex process of visual imagination. “By expanding our understanding of the circuits governing mental simulation in a cognitively healthy subject, we will also gain insight into neuropsychiatric conditions like PTSD and hallucination, where a patient may be unable to distinguish between real and simulated experiences or lose control of internally created sensations,” Sheinberg said.
PROJECTS TACKLING ALZHEIMER’S DISEASE AND RELATED DEMENTIAS
Drug discovery for the prevention and treatment of Alzheimer’s disease
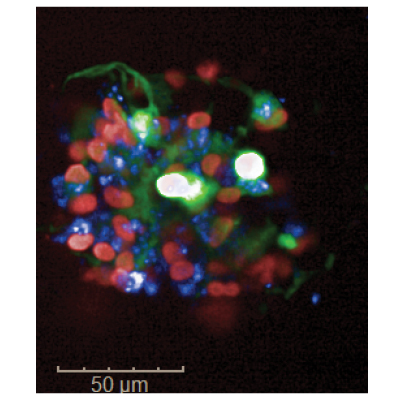
high-throughput confocal microscope with nuclei shown in red and
amyloid plaques in blue.
Investigators: Robbert Creton, professor of medical science (research), and Jill Kreiling, associate professor of molecular biology, cell biology and biochemistry (research)
There is an urgent need for more rapid preclinical testing of a novel class of Cyclosporine A type drugs, a recently discovered class of drugs that researchers believe may have applications for preventing and treating Alzheimer’s disease. Creton, Kreiling and their labs will study the impact of these drugs on 3D cultures of human neural stem cells engineered to form amyloid plaques and tau tangles, the proteins believed to negatively affect neurons in Alzheimer’s disease. To analyze their results, the team will utilize custom-developed high-throughput methodologies for imaging, automated image analysis and analyses of complex signaling networks. “This project is too risky for external funding at this time because Cyclosporine A type drugs have not been tested in preclinical Alzheimer's models. However, once we show that these drugs work in human neurons, it will be very appealing because Cyclosporine A type drugs are already in use for other conditions and may be repurposed as therapeutics for Alzheimer's disease," the team explained.
Metabolomic profiling in the locus coeruleus during stress and neurodegeneration

Neuroscience. From left: Eric Morrow, Qing Ouyang, Gajendra Kumar,
Eugene Lee and Li Ma.
Investigator: Eric Morrow, Mencoff Family Professor of Biology and Director of the Center for Translational Neuroscience
As the primary source of noradrenaline for the brain, the locus coeruleus is a neuromodulatory system regulating cognition, sleep and other behaviors. Pathology in the locus coeruleus is also one of the earliest events in Alzheimer’s disease, preceding and correlating with cognitive decline. The Morrow Lab will study a newly discovered metabolic pathway governing early locus coeruleus vulnerability that is relevant to Alzheimer’s disease and sleep. “If successful, our work will build a foundation for the development of inexpensive, widely-accessible, nutritional supplements that promote locus coeruleus health, and thereby may delay or prevent neurodegeneration,” said Morrow.
Development of a dual-bead-based immunoassay for the detection of Alzheimer’s disease biomarkers in neuronal-derived extracellular vesicles
Investigator: Anubhav Tripathi, professor of engineering, professor of biology and medical sciences
Researchers are able to screen neuronal-derived extracellular vesicles, particles that are naturally released from neurons and which are found in most biofluids including blood, for Alzheimer’s disease biomarkers. However, there is currently no method for screening extracellular vesicles for Alzheimer’s disease biomarkers quickly enough and at a high enough volume to make it a practical way for diagnosing patients. Through the innovation award, the Tripathi Lab will develop a dual-bead-based immunoassay for the detection of Alzheimer’s disease biomarkers in neuronal-derived extracellular vesicles. The lab has already developed an ultrasensitive method of protein detection and quantification for amyloid-beta, one of the proteins believed to negatively affect neurons in Alzheimer’s disease. Next, they will develop a multiplex capability and automate the process of sample preparation and cleaning. “We hope that our platform not only has a higher sensitivity than current methods of biomarker detection, but that it can provide a more user-friendly and cost effective solution for blood sample analysis. We are focusing on lowering the amount of sample required for the analysis of extracellular vesicles, which should increase turnaround time and provide patients and their families with faster results,” the team said.



