Obsessive Compulsive Disorder, commonly referred to as OCD, is a psychiatric illness marked by obsessions (recurrent unwanted or distressing thoughts) and compulsions (repetitive, ritualistic behaviors). OCD affects approximately 2 percent of the U.S. population, and 10-20 percent of cases are resistant to treatments.
However, Deep Brain Stimulation (DBS) in the ventral capsule/ventral striatum has been found to improve symptoms in approximately 50-70 percent of patients. Early trials of DBS have been promising, but clinical trials have failed to date. These failures may be attributed to the “open-loop” nature of DBS, where stimulation parameters are chosen during infrequent visits to the clinician’s office. The continuous stimulation fails to address the dynamic nature of OCD, and symptoms often fluctuate over minutes to days.
Titrating DBS to respond to symptoms as they arise—such as “Adaptive DBS”—may be a more effective approach for treating symptoms of OCD and reducing undesirable side effects of stimulation, according to research from Carney-affiliated faculty David Borton, Michael Frank and Matthew Harrison.
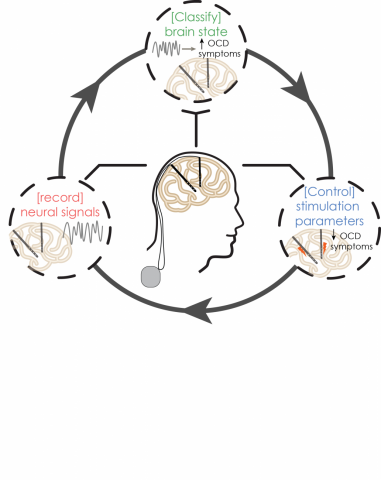
In a closed-loop, adaptive system:
- Electrodes would continuously record electrical activity from the brain;
- Recorded data would be used to classify maladaptive mental states as they arise;
- Stimulation parameters would be adjusted accordingly to relieve symptoms.
This closed-loop paradigm relies heavily on stable, definitive biomarkers of maladaptive mental states. However, electrophysiological biomarkers and corresponding optimal stimulation parameters required for such an adaptive system are currently not well defined.
In this ongoing NIH-funded study, 10 OCD patients will be implanted with DBS devices, which consist of an implantable pulse generator that connects to two electrode leads that are implanted in the brain. These electrodes are capable of both recording from and stimulating the brain.
The first participant underwent surgery in October 2018, and recruitment is ongoing. Participants will return on a biweekly to monthly basis over the course of two years for clinical evaluation and testing during behavioral tasks, which offer a unique opportunity to identify biomarkers and optimal stimulation parameters in controlled settings.