E. Javier López Soto, a postdoctoral research associate in Brown University’s Lipscombe Lab, was discussing an experiment with an undergraduate student in March when he received a long-awaited phone call from the Warren Alpert Foundation.
An unsettling feeling immediately took over his body. His mind focused on a lingering question: would he receive the foundation’s fellowship in neuroscience to which he had applied in January?
“I was already checking their website every day,” López Soto said. “I just wanted to know.”
When the man on the other end said, “we want to give you the funding to pursue your research,” López Soto became speechless. “I wasn’t expecting it, so I didn’t know what to say,” he recalled. “You never think that you’re actually going to get it.”
López Soto is one of five neuroscientists nationwide to receive the prestigious fellowship, which is given as a transitional post-doctoral award to scientists before they become full-time faculty. He will receive $200,000 annually for two years to cover salary, lab costs, and related expenses. Brown nominated López Soto for the award after a competitive internal application process
“This is such an important award for Javier and so well deserved,” said Diane Lipscombe, director of the Lipscombe Lab and of the Carney Institute for Brain Science. “Javier is a great scientist, creative, and extremely dedicated. I’m thrilled that he will be able to continue his exciting research with me, while also transitioning toward developing his own independent research program.”
From electrophysiology to solving scientific mysteries
As a graduate student at National University of La Plata in Argentina, López Soto focused on electrophysiological methods to study the μ-opioid receptor gene in humans. Four years ago, after finishing his Ph.D. in neuroscience, López Soto joined the Lipscombe Lab, which studies voltage-gated calcium channels and how calcium ion channel expression regulates specific neuronal functions and contributes to nervous system disorders, such as chronic pain, migraines, ALS, and psychiatric illness.
“When I joined Diane’s lab, my idea was to expand my skills to different areas and learn new techniques,” said López Soto, who is now highly skilled in bioinformatic, genetic, molecular, and behavioral techniques and analyses.
After moving to the United States, López Soto immediately noticed major differences in access to resources for research between the U.S. and Argentina. In Argentina, he said, there is a supply shortage of reagents and equipment and less funding for scientific research.
“Limitations encourage creativity and resourcefulness, and I believe these challenges have made me a better scientist,” he said.
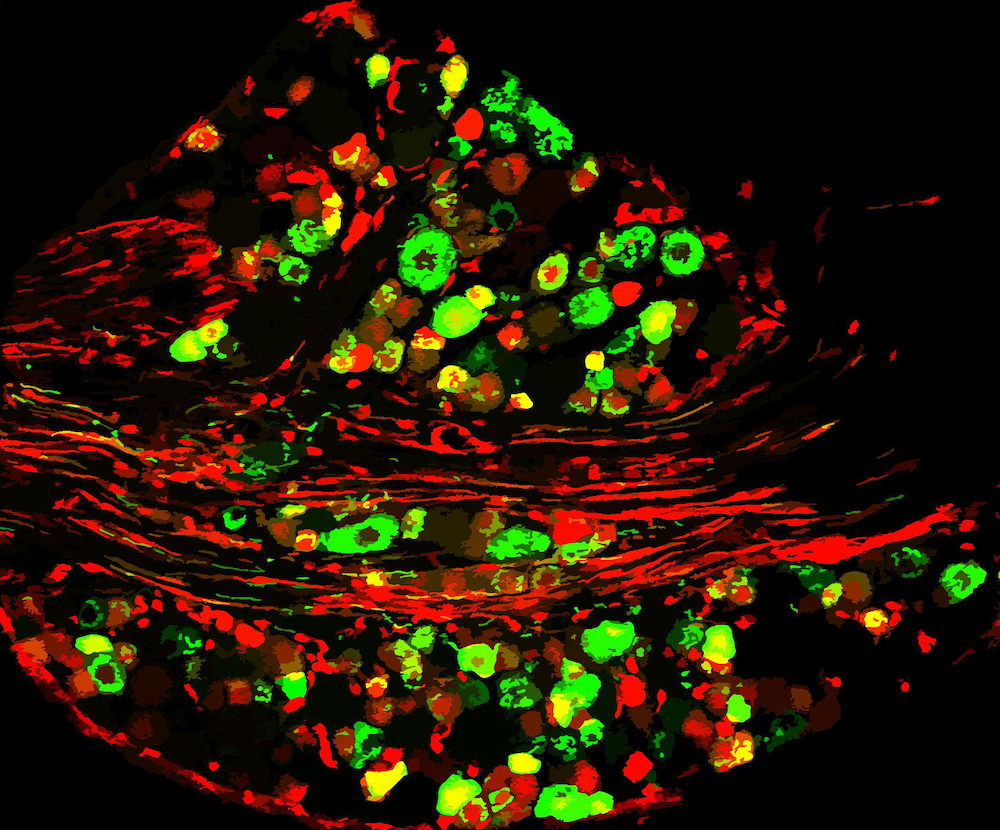
Through experiment and resourcefulness, López Soto solved a mystery that eluded the Lipscombe Lab for many years: the molecular mechanism that drives cell-specific exon-selection in the voltage-gated calcium channel in nociceptors. Nociceptors are sensory receptors that send signals which cause the perception of pain.
“We discovered that a DNA-binding protein, when bound to a specific region of the calcium channel gene, regulates the final composition of calcium channels expressed in nociceptors,” López Soto said. “The binding of this protein is itself regulated by epigenetic factors.”
Developing alternative therapies to treat pain
Presynaptic voltage-gated calcium channels and their associated proteins are targets of major analgesics to treat neuropathic pain. However, current painkillers, including those that target calcium ion channels, lack broad efficiency, have major side effects, and many—like opioids—are addictive and have contributed to the opioid epidemic nationwide.
“When you think about pain management, calcium channels come to mind because they are the gatekeepers of transmission of pain signals from all parts of the body to the brain,” López Soto said. “Inhibitors of certain calcium channels are very effective analgesics, but they also come with a lot of side effects from blocking the activity of calcium channels found in many other neurons, in addition to nociceptors.”
The Lipscombe Lab has demonstrated that nociceptors express a form of the calcium channel which is slightly different in composition and in its sensitivity to drugs like morphine, compared to other neurons. López Soto recently discovered the basic mechanisms that control the expression of these different calcium channel forms.
The Warren Alpert Foundation fellowship award will allow López Soto to continue researching key molecular remodeling events in sensory neurons that accompany nerve damage and that are associated with neuropathic pain. He said all constructs, tools, and databases developed during this study will be available to other scientists.
López Soto’s ultimate goal, he said, is to inform the development of new non-opioid related therapies to mitigate neuropathic pain.
“New therapeutic strategies are desperately needed to treat chronic pain, and this motivates my project,” he said. “I’m really excited to learn how to do massive RNA sequencing and to use next-generation technologies to define the precise composition of RNAs expressed in nociceptors. The award gives me a nice platform to do this.”
López Soto will present his research at a mini-symposium at the Society for Neuroscience’s annual meeting in Chicago in October.
“Javier,” Lipscombe said, “has a great future.”
Visit López Soto’s profile for a list of research projects and published papers.
