Announcements
NIH announces Ruth L. Kirschstein National Research Service Award (NRSA) Stipends, Tuition/Fees and Other Budgetary Levels Effective for Fiscal Year 2024 (4/23/24)
From the NIH's Open Mike blog "we are pleased to announce stipend and childcare subsidy increases for the over 17,000 early career scholars supported on NIH Kirschstein National Research Service Awards (NRSAs) (NOT-OD-24-104). Stipends will be raised by 4% for predoctoral trainees and by 8% for postdoctoral scholars in fiscal year (FY) 2024 compared to last year), the most substantial year over year increase since FY 2017. Additionally, the childcare subsidy will be increased by an additional $500 (from $2500 to $3000) in FY24". Note that the budgetary categories described in Notice NOT-OD-24-104 apply only to Kirschstein-NRSA awards made with FY 2024 funds. All FY 2024 awards previously issued using NOT-OD-23-076 will be revised to adjust funding to the FY 2024 levels.
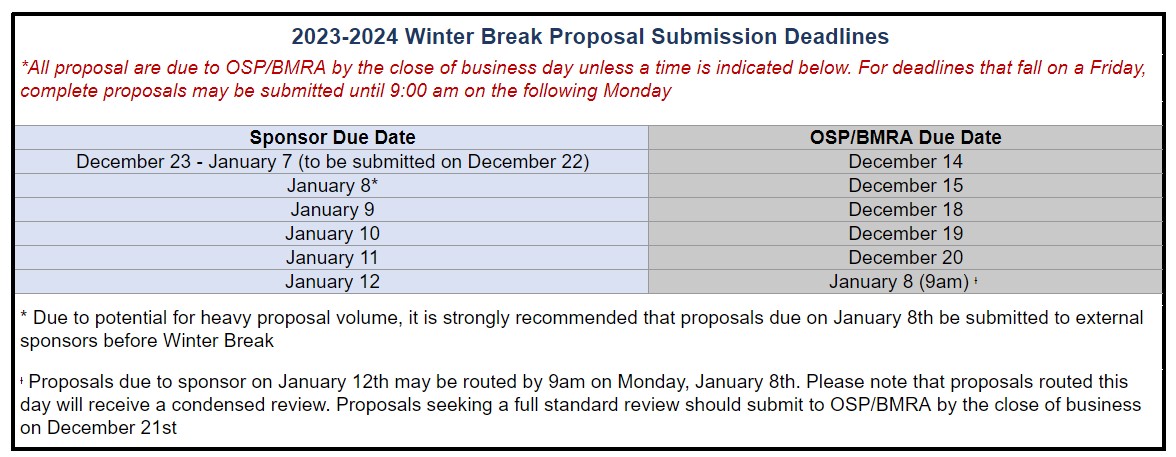
2023/24 Winter Break Proposal Submission Deadlines (10/25/23)
Upcoming Proposal Submission Deadlines for OSP/BMRA Review (9/7/23)
SciENcv Required for NSF Biosketches and Current & Pending after October 23, 2023 (8/18/23)
As required by NSF's PAPPG 23-1, Biosketches and Current & Pending documents submitted on or after October 23, 2023 must be created using SciENcv. To assist with this requirement, OSP has partnered with University Library to provide additional information on the SciENcv system. This guidance is intended for both faculty and administrative staff who help facilitate proposal submission.
Below please find the slides and video from our recent training session on August 17th, 2023.
Upcoming Proposal Submission Deadlines for OSP/BMRA Review (4/13/23)

NSF Safe and Inclusive Working Environments Plan for Off-Campus or Off-Site Research [Jan 2023] (2/24/23)
The NSF Proposal & Award Policies & Procedures Guide (PAPPG) was updated to incorporate Safe and Inclusive Working Environments for Off-Campus or Off-Site Research language (Chapter II.E.9) which describes the new requirement for the Authorized Organizational Representative (AOR) to certify that Brown has a plan in place for safe and inclusive research for any proposal that proposes to conduct off-campus or off-site research. Beginning in March 2023, several solicitations from BIO and GEO will require the submission of a Safe and Inclusive Working Environments Plan that will be considered as part of the Broader Impacts criteria during the review process. For all NSF applications proposing off-campus or off-site research, the University requires a copy of the Plan at proposal submission stage to ensure that the application package is complete in the event an award is made. More details can be found here.
New NSF Biosketch and Current & Pending Forms Required As Of January 30, 2023 (1/25/23)
Please be aware that NSF's new Proposal & Award Policies & Procedures Guide (PAPPG) goes into effect on Monday, January 30th. One of the notable changes is an update to the agency's Biosketch and Current & Pending documents, with a new requirement that these forms be certified by PI. The certification step is required for forms created via SciENcv and/or NSF fillable forms. Note that these forms are certified by the PI typing their name into the appropriate field; digital signatures are not allowed.
Please see the links below for the new NSF fillable forms. Please note that these fillable forms will only be available until October 22nd, 2023. After that date, all Biosketches and Current & Pending documents must be prepared in SciENcv. We encourage investigators to begin using SciENcv for these forms as soon as possible in order to assure a smooth transition when this requirement takes effect.
- NSF Fillable Biographical Sketch for proposals submitted on or after January 30, 2023
- NSF Fillable Current & Pending for proposals submitted on or after January 30, 2023
Upcoming Proposal Submission Deadlines for OSP/BMRA Review (1/20/23)
The DHHS/NIH Salary Cap has been increased effective on January 12, 2023 (1/19/23)
The maximum salary that can be awarded and charged to a sponsored project will increase from $203,700 to $212,100. Please see the NIH notice (NOT-OD-23-056) for more details. Brown's NIH Salary Cap Worksheet has been updated to reflect the increase. Please contact your OSP Representative if you need any further information.
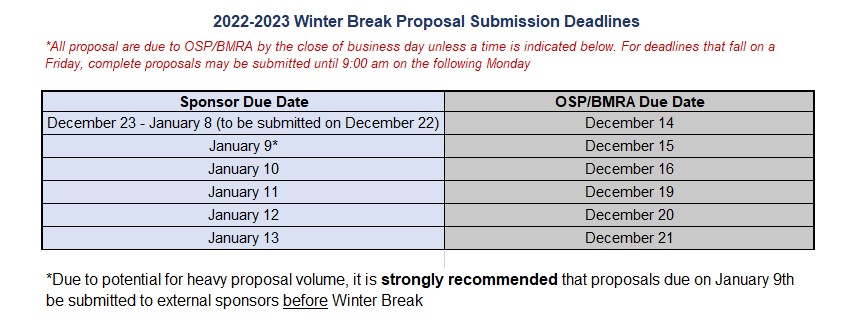
Winter Break Proposal Submission Deadlines (10/19/2022)
Upcoming Grants.gov Downtime, September 23-29, 2022 (8/17/22)
Grants.gov has a planned production system outage from Friday, September 23, 2022 at 12:01 AM ET to Thursday, September 29, 2022 at 11:59 PM ET (see Grants.gov calendar). Grants.gov will use this time to migrate their services to the Cloud. During this downtime, Grants.gov will be unable to receive proposal submissions and agencies will be unable to post new Funding Opportunity Announcements.
In response, NIH and AHRQ due dates that fall on or between September 22 and September 30, 2022 will move to October 3, 2022.
Note that individual sponsor systems, such as ASSIST, eRA Commons, and Research.gov will remain online, with only the proposal submission function being unavailable. Other actions and tools available in those systems that do not utilize Grants.gov will not be impacted.
For additional clarification on how this downtime will affect NIH Continuous Submission proposals, NIH Late Submissions, and in-progress Workspace proposals, please see the links below:
What to Expect During the Grants.gov Cloud Migration and Extended Downtime for September 2022
Adjustments to NIH and AHRQ Grant Application Due Dates Between September 22 and September 30, 2022
Congress Strengthens NIH’s Ability To Address Harassment in NIH-funded Activities (6/8/2022)
Effective July 9, 2022 NIH is implementing a general provision in the 2022 Consolidated Appropriations Act (P.L. 117-103) that makes reporting to NIH by NIH-funded institutions mandatory “when individuals identified as a principal investigator or as key personnel in an NIH notice of award are removed from their position or are otherwise disciplined due to concernsabout harassment, bullying, retaliation, or hostile working conditions.” The Institutional Reporting Process is outlined here. For guidance on this topic published by other federal agencies, please refer to this web page.
OSP/BMRA Revised Proposal Submission Due Dates for Spring/Summer Holidays (5/18/22)
Recently it was announced that Brown University will be granting staff additional holidays. In order to adhere to the Proposal Submission Policy & Guidelines, proposals will have revised dues dates to OSP/BMRA to ensure a full and thorough proposal review is conducted. Please see the attached calendar for the revised due dates.
Please note that proposals due for the NIH June 5th and July 5th deadlines do have revised due dates to OSP/BMRA. The due dates for proposals due during or directly after winter break are still being determined and will be announced near the start of the Fall 2022 semester.
The Department of Energy (DOE) offers Supplemental Funding for collaborations with Ukrainian Scientists and Students (4/4/22)
DOE's Office of Science (SC) published a Dear Colleague letter encouraging university principal investigators who currently receive financial assistance from SC to consider requesting supplemental funds to host or collaborate with students or scientists who have been impacted by the war in the Ukraine. Through these supplements, SC aims to protect the well-being and livelihood of students and scientists impacted by the war by maintaining strong connections to the worldwide scientific community. You can read the DOE full letter including application instructions here.
New DHHS Salary Cap (2/16/22)
The DHHS/ NIH Salary Cap has been increased effective on January 2, 2022. The maximum salary that can be awarded and charged to a sponsored project will increase from $199,300 to $203,700. Please see the NIH notice (NOT-OD-22-076) for more details. Brown's NIH Salary Cap Worksheet has been updated to reflect the increase. Please contact your OSP Representative if you need any further information.
New Federal Minimum Wage Regulation effective January 30, 2022 (1/5/2022)
“Increasing the Minimum Wage for Federal Contractors” Final Rule has been published in the Federal Register. The new regulations require government contractors (including Brown University) to pay certain individuals working on or in connection with government contracts $15 per hour starting on January30, 2022. The requirement generally applies to contracts effective January 30, 2022 or later. The requirement does not apply to grants, certain student workers, or individuals performing on covered contracts for less than 20 percent of their work week. Questions on whether or how to budget for this federal requirement should bedirected to your OSP Grant & Contract Administrator.
Proposal Submission *NEW* 2022 Requirement (1/5/2022)
Effective immediately, all proposals with submissions due before 5 pm Eastern time must identify the prior day as the deadline. In Coeus use the Mailing Info Tab to identify the due date and add a comment regarding the time. OSP relies on this information to triage applications for action by due date. Please let us know of any questions.
_________________________
ARCHIVED ANNOUNCEMENTS
COVID-19 Related Notices
COGR | NIH | NSF | COVID-19 RELATED ANNOUNCEMENTS | OPERATIONAL TRAVEL CHANGES
On June 16, 2020, most of the administrative flexibilities described in the Office of Management and Budget (OMB) Memos M-20-17 and M-20-20 expired. Of immediate interest please take note of the following changes:
Travel
The ability to charge cancellation fees associated with project specific travel were included in these flexibilities. Any cancellation fees incurred on or after June 17 may no longer be charged to a sponsored project. Please note that university travel restrictions remain in place.
Proposal Applications and Research Performance Progress Report (RPPR)
Proposal applications and RPPR reports are due on the normal established deadline schedule unless a specific exception has been granted by the agency.
OMB Memo M-20-11 will expire on July 26, 2020, this page will be updated as further agency implementation plans are released.
COGR
COGR - Agency information (5/6/20)
COGR - Institutional Information (5/6/20)
COGR - FAQs (5/6/20)
NIH
NIH Implementation of OMB Memorandum M-20-26 "Extension of Administrative Relief for Recipients and Applicants of Federal Financial Assistance Directly Impacted by the Novel Coronavirus (COVID-19) due to Loss of Operations" (6/29/20)
Applicant/Recipient COVID-19 Update History (4/2/20)
Archived NIH COVID-19 notices
NSF
Coronavirus Information
NSF Implementation of OMB Memorandum M-20-26 "Extension of Administrative Relief for Recipients and Applicants of Federal Financial Assistance Directly Impacted by the Novel Coronavirus (COVID-19) due to Loss of Operations" (6/29/20)
Archived NSF COVID-19 notices
COVID-19 RELATED ANNOUNCEMENTS
NIH FOREIGN COMPONENT NOTIFICATION | RESEARCH PERSONNEL EFFORT |
PRG 300 COVID-19 PROGRAM WORKTAG | EFFORT REPORTING - COVID-19 INTERIM PROCESS
NIH FOREIGN COMPONENT NOTIFICATION
NIH Foreign Component notification requirement found in the NIH COVID Flexibilities FAQs here , please note the IMPORTANT 5/21 REVISION to the FAQ below:
For post-docs that are required to work on their originally approved work remotely from a foreign country due to COVID-19 travel restrictions, where no grant funds are going to a foreign entity, NIH has determined that this scenario does not constitute the performance of a significant scientific element or segment of the project outside the US, as outlined in the NIH Grants Policy Statement definition of a foreign component.
This FAQ response is an update to an email message sent at the end of April to all active NIH PIs.
Should you have any questions or concerns, please contact your OSP Representative or your BMRA Contact.
-
RESEARCH PERSONNEL EFFORT
Through June 16, 2020 many Federal Agencies (e.g., NIH, NSF, NASA, ONR, DARPA) allowed grant/cooperative agreement recipients to continue to charge salaries and trainee stipends to currently active Federal awards consistent with the recipients' policy of paying salaries (under unexpected or extraordinary circumstances) from all funding sources, Federal and non-Federal. This included researchers who were unable to work as a result of or related to the effects of COVID-19 in accordance with university policy.
If progress of the work has been or will be impacted, PIs should consult with their program officer to determine if a scope of work change, time extension or other action is necessary. If so, the submission of an agency notification is done through OSP or BMRA.
The extension of these emergency federal regulations beyond mid-June - is under assessment by the University. OSP will be posting further guidance as it becomes available.
-
PRG 300 COVID-19 PROGRAM WORKTAG
In April Workday Communications notified the community of a new Program Worktag to track expenses related to the ongoing COVID-19 pandemic. We encourage use of the PRG300 COVID-19 for sponsored project funds as well. Federal Agencies allowed salaries, fringe benefits during the work remote order and the repurposing of grant funds including supplies and personnel through June 16, 2020, it is critical that Brown be able to track these and any other financial impacts on internal and external funds.
Repurposing Existing Federal Financial Assistance Programs and Awards (Grants) to Support the Emergency Response to the Novel Coronavirus (COVID-19)
NIH, NSF and NASA Grant Recipients authorization to donate PPE and other research supplies to support COVID-19 efforts (e.g., to hospitals and local health care facilities) expired on June 16, 2020.
-
COVID-19 Effort Reporting Interim Process
The purpose of this Notice is to alert the community of administrative flexibilities that will apply/be adopted under the University's Effort Certification Policy during the Provost's Remote Work order period related to the COVID-19 crisis.
OSP has identified the following short-term administrative flexibilities to assist our Effort Certification Partners and Certifiers in managing the monthly effort reports during the remote work order period.
Schedule and Deadlines
-
Effort reports for undergraduate and non-exempt employees will be completed on a quarterly basis for the prior 90 day period, rather than on a monthly basis to reduce the administrative burden.
-
A 30 day extension will be granted for effort certification reporting periods impacted by the remote work order.
The schedule for the non-exempt and undergraduate effort report will be as follows:
|
Effort Reporting Period |
Reports Generated |
Certification Deadline |
|
|
|
|
|
March 1, 2020 – March 28, 2020 |
May 8, 2020 |
July 7, 2020 |
|
|
|
|
|
March 29, 2020 – April 25, 2020 April 26, 2020 – May 30, 2020 May 31, 2020 – June 27, 2020 |
August 7, 2020 |
October 6, 2020 |
|
|
|
|
|
June 28, 2020 – July 25, 2020 July 26, 2020 – August 29, 2020 August 30, 2020 – Sept 26, 2020 |
November 9, 2020 |
January 8, 2021 |
|
|
|
|
|
Sept 27, 2020 – Oct 31, 2020 Nov 1, 2020 – Nov 28, 2020 Nov 29, 2020 – Dec 26, 2020 |
February 9, 2021 |
April 10, 2021 |
Semi-annual effort reports will be generated on August 7, 2020 and February 9, 2021 as originally planned, with the certification deadlines coinciding with the extended schedule of October 6, 2020 and April 10, 2021, respectively.
Many Federal agencies and other sponsors have released guidance related to award management and the impacts of COVID-19. OSP will be reviewing this guidance and assessing its impact on our effort reporting processes. In the meantime, please continue to follow all relevant policies and procedures and apply those practices consistently.
Effort Certification Statement
For a limited period of time in 2020 many Federal Agencies (e.g., NIH, NSF, NASA, ONR, DARPA) are allowing grant/cooperative agreement recipients to continue to charge salaries and trainee stipends to currently active Federal awards consistent with the recipients' policy of paying salaries (under unexpected or extraordinary circumstances) from all funding sources, Federal and non-Federal. This includes researchers who are unable to work, or are “idle”, as a result of or related to the effects of COVID-19 in accordance with university policy. With Federal Agencies allowing salaries, fringe benefits during the work remote order and allowing the repurposing of grant funds, it is critical that Brown be able to track the financial impact on internal and external funds during this time. Please use the COVID-19 worktag PRG300 COVID-19 when tracking such expenses for sponsored project funds.
Please note that the effort certification language that researchers will certify on the effort report will be modified to acknowledge that effort may include periods of little or no work, but that payroll charges will continue as allowable under sponsor guidance.
Example: I acknowledge that the effort report may include periods of little or no work in accordance with the University’s job continuity policy and as allowable under Federal regulations and sponsor guidance.
-
OPERATIONAL TRAVEL CHANGES DUE TO COVID-19
For non-refundable tickets purchased prior to March 9, 2020, our current guidance is that cancellation fees on federal grant related activities can be charged to sponsored funds if the cancellation was a result of an external organization’s action, (e.g. a vendor cancelled a conference or site visit), is due to the CDC travel restrictions (see CDC Level 3 countries) or is due to Brown’s prohibition on travel. The authority to charge cancellation fees to federal projects ended June 16, 2020, with the expiration of OMB Memo M-20-17.
Departmental Cost Center Managers/Authorized Approvers must ensure any change or cancellation fees charged to sponsored funds are reasonable and allowable. Travelers should retain documentation of their cancellation request, change/cancellation fees and remaining vendor credits.
Please note that Trip Cancellation Insurance is not ordinarily allowed on sponsored awards. At this point in time, the preferred action is purchase of a refundable ticket. For non-federal sponsors, the award’s terms & conditions will require review as to allowability. Contact your OSP Staff Grant & Contract Administrator or Accountant with questions on non-federal awards.
About OSP
The Office of Sponsored Projects (OSP) supports Brown University faculty, students and staff in the acquisition, performance, and administration of projects and programs funded by external sources. OSP provides the University's central coordination and oversight of research by offering a wide range of services including review and submission of proposals; award negotiation and acceptance; dissemination of research policy information to campus; issuance of subaward agreements and sub-recipient monitoring; compliance with governmental and private funding agency standards; advising on financial management of sponsored projects, financial and expense reporting; cash management; effort reporting, and monitoring of cost-share arrangements; addressing financial and administrative issues that arise during the life of a sponsored project; coordination of award close-out process; and providing education and professional development opportunities in research administration for the campus community.
Pre-Award Staff are responsible for:
|
Post-Award Staff are responsible for:
|
|
|