
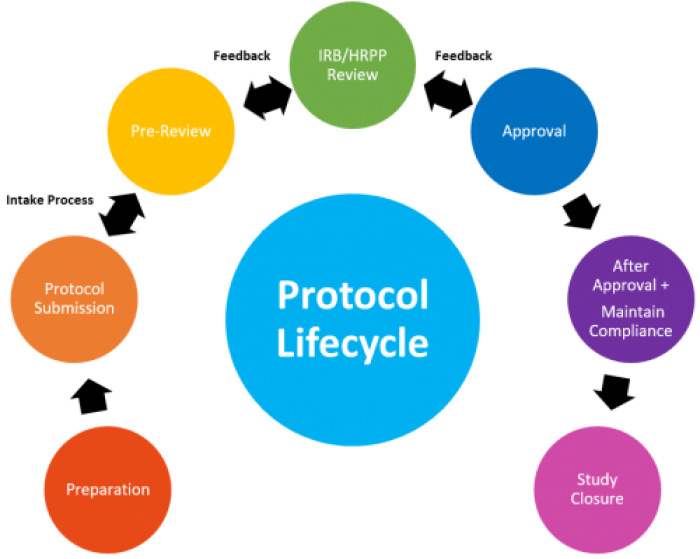
PREPARATION
|
SUBMISSION
|
|
REVIEW QUEUE
|
REVIEW PROCESS
|
AFTER APPROVAL
|
STUDY CLOSURE
|
FAQs
|
REVIEW LIFECYCLE
DO YOU NEED IRB REVIEW?
Refer to the guidance and helpful information on the HRPP Does My Project Need IRB Review? and Forms and Templates webpages.
There are many ways to get in contact with HRPP for assistance on making this determination. See ways to contact HRPP in the FAQs section of this page, HRPP/IRB Home Page webpage, or the Contact the HRPP webpage.
Your study meets the definition of human subjects research and you would like to submit to Brown’s IRB/HRPP.
-
Step 1: Review the HRPP Principal Investigator (PI) Eligibility & Advisor Qualifications webpage to ensure you meet Brown’s PI eligibility requirements.
-
Step 2: Are you new faculty coming to Brown? Review the OVPR PI Transitions – Incoming Faculty webpage to about the recommendations and requirements for transferring awards, submitting new proposals before your arrival, transferring data and/or materials, conducting human subjects research, export controls, and conflict of interest.
-
Step 3: Are you new to human subjects research at Brown? Sign up for a one-on-one New Investigator/Research Staff Onboarding Session to learn about the IRB/HRPP website, forms, processes, and policies.
-
Step 4: Refer the HRPP Education Instructions and FAQs webpage and complete all the human subjects research education applicable to your study.
-
Step 5: Review the HRPP Ancillary Reviews webpage to consider if any other department or committee at Brown may need to review your study, as this could impact how long it takes before you may begin research activities.
-
Step 6: Review the HRPP Collaborative Research webpage if your study will be conducted at any location other than Brown (domestic or foreign) and/or with non-Brown investigators. Your study may require a reliance agreement, which could impact how long it takes before you may begin research activities.
-
Step 7: Refer to the HRPP homepage for Review Times and Full Board Meeting Dates & Submission Deadlines. Review Times display the latest submission under review by the IRB/HRPP. These dates are updated each Friday. The Full Board Meeting & Deadline Dates list the IRB’s monthly meetings, the protocol submission deadlines, and pre-review response deadlines. These dates and deadlines are only applicable to greater than minimal risk research or other situations recommended by HRPP staff. There are no submission deadlines for minimal risk research.
-
Step 8: See submission instructions on the HRPP Forms and Templates webpage for submission instructions; applications; application guidance; consent/assent templates and HIPAA Authorization template; continuing review and closure forms.
- Step 9: Review the IRB Guidance & Policies webpage to ensure IRB’s applicable established guidance and policies are incorporated into your study appropriately.
- Step 10: See the Huron System Guidance available on the HRPP Huron IRB Electronic Submission System webpage. This guidance covers how to submit a new study, modification, continuing review, closure, and more in the Huron System .
See submission instructions on the HRPP Forms and Templates webpage, the Huron Lifecycle of a Study Guidance webpage for more detailed Huron information, and then submit in the Huron
IRB Electronic System.
If you have any questions there are many ways to get in contact with HRPP. See ways to contact HRPP in the FAQs section of this page, HRPP/IRB Home Page, or the Contact the HRPP webpage.
Review dates can be found on the IRB/HRPP Home Page. The submission remains in the queue and in Huron Pre-Review until assigned for review by an IRB Coordinator. Review queue timelines depend on current submission volume.

What is the holistic review process? The IRB/HRPP will conduct a holistic review of your active study with each submission as part of the post-approval monitoring process to ensure it remains in compliance with current federal regulations, Brown policies, and IRB position statements.
What is the 45 Day Review Policy? If a submission is not in approvable condition within 45 calendar days of the first feedback sent by HRPP, then the submission will be withdrawn.
Minimal Risk Research (Exempt/Expedited) - new or modification
- HRPP will reach out via Huron with Clarification Requested that includes any pre-review feedback that needs to be addressed prior to IRB review, if required. You will access the feedback and respond in the Huron System, but receive an email message when Clarifications are being requested. This pre-review process is intended to assist with a streamlined IRB/HRPP process by attending to issues that may delay IRB/HRPP approval.
- Once the research team addresses feedback and the submission is set back to Pre-Review in the Huron System, the HRPP pre-reviewer may have follow up Clarification Requested based on the responses. This process may repeat depending how much new information is shared by the research team, how many complex questions need to be addressed, etc.
- Once all pre-review feedback is addressed, an IRB member or HRPP staff member provides a final review of the submission.You can see this in the Huron Workflow when the submision moves to IRB Review. The IRB member or HRPP staff member may provide additional clarifications that needs to be addressed by the research team prior to approval.
- You can follow the workflow of Pre-Review, IRB Review, Clarifications Requested, and Post Review in the Huron System.
- Once the submission has no regulatory concerns, it moves into Post Review. The IRB Coordinator finalizes documents and sends out the approval memo.
- Once all approval documents are received by the research team, the submission moves to Review Complete.
NOTE: Once the review begins direct all review related questions/emails/phone calls/Huron comments to the assigned IRB Coordinator and no longer include the main IRB inbox on correspondence.
Greater than Minimal Risk Research (Full Board) - new or modification
There is one Full Board meeting a month. The submission deadlines for review at each meeting and meeting dates are available on the HRPP/IRB Home Page.
- Once a submission in Pre-Review in the Huron System, HRPP staff determine if it requires Full Board review. Once this is determined submissions are assigned to an IRB Coordinator for pre-review, so the submission is ready for review at that month’s Full Board meeting.
- Once Pre-Review feedback is provided via Huron with Clarification Requested, you will access the feedback and respond in the Huron System. You will receive an email message from Huron when Clarifications are being requested. This pre-review process is intended to assist with a streamlined IRB process by attending to issues that may delay IRB approval.
- Be mindful of the pre-review response deadline that is noted on the HRPP website and in the Clarifications Requested. If the Clarifications Requested are not sufficiently addressed prior to the response deadline, the submission may not be reviewed at the Full Board meeting and moved to IRB Review in the Huron System. If all pre-review comments are addressed, the submission is assigned to the Full Board meeting agenda.
- The Thursday before the meeting the Board receives all materials for their review.
-
A determination is provided at the Full Board meeting.
- It is recommended that the PI be available during the meeting in case the Board has any questions that need clarification by the PI. This is not a requirement.
- HRPP will communicate via Huron Post-Review and/or Huron Comment on the Board’s determination after the meeting.
Potential determinations and next steps:
-
Approved (No changes are required):
- The research team will receive an approval memo and finalized documents in the Huron System when the submission moves to Review Complete.
-
Contingently approved (Minor, specific, non-substantial modifications are required):
-
The research team will be informed by email with a status change in the Huron System, and then receive a contingent approval memo with the Board’s requests up to a week later via the Huron System.
- The PI is then responsible for addressing all requests and following next steps provided in the memo.
- Once all non-substantial modifications and questions are sufficiently addressed, the research team will receive an approval memo and finalized documents in the Huron System.
- The submission will then move to Review Complete.
-
The research team will be informed by email with a status change in the Huron System, and then receive a contingent approval memo with the Board’s requests up to a week later via the Huron System.
-
Deferred (Substantial modifications and/or additional information are required):
-
The research team will be informed via the Huron System Post-Review notice and/or Huron Comment, and then receive a memo with the Board’s requests up to a week later via Huron System.
- The PI is then responsible for providing responses and modifications to the material requested within the timeline outlined on the memo, if the PI intends to re-submit and receive IRB approval for the study
- Once all substantial modifications are addressed, the submission will be reviewed again at the closest Full Board meeting and given a new determination. Depending on the new determination, please see above for more information.
-
The research team will be informed via the Huron System Post-Review notice and/or Huron Comment, and then receive a memo with the Board’s requests up to a week later via Huron System.
NOTE: Once review begins direct all review related questions/emails/phone calls to the assigned IRB Coordinator and no longer include the main IRB inbox on correspondence.
After you receive IRB/HRPP approval, you may begin any research activities not being held up by ancillary reviews. If you need to make changes to the approved study you should to submit a modification in the Huron System prior to implementing any changes, and the review cycle will start again.
- Step 1: Review the HRPP Clinical Trials webpage if your study meets the federal definition of a clinical trial. This webpage will guide you through your responsibilities with registration and reporting requirements. The ClinicalTrials.gov specific registration webpage also provides helpful information.
- Step 2: Refer to the IRB Policy on Reportable Events and Noncompliance if your study has a qualifying event. All Reportable Events are submitted in the Huron System as Reportable New Information (RNI).
- Step 3: Refer to the HRPP Forms & Templates webpage for the Continuing Review Form and the Huron System Guidance for Continuing Reviews if you are required to submit continuing reviews.
- Step 4: Consider signing up for the QA/QI Administrator’s Best Practice Reviews for individualized support and guidance to assist with protocol maintenance and ensure compliance. If you prefer self-guided post-approval monitoring, use the Best Practice checklist as an independent guide to confirm compliance.
Refer to the HRPP Forms & Templates webpage for the Study Closure Form and the Huron System Guidance for Closures when your study is complete, and you are ready to close it.
Are you an investigator leaving Brown and your study is not ready for closure? Review the OVPR PI Transitions – Outgoing Faculty webpage to about the recommendations and requirements for transferring subawards, equipment, data, materials, research, licenses, etc. before you leave Brown.
See the Research Data Management Offboarding Checklist and webpage for Brown University policies surrounding closing a research study here at Brown.
FREQUENTLY ASKED QUESTIONS (FAQ)
What if I don’t know if my project requires IRB/HRPP review?
How long does it take for my study to receive IRB/HRPP approval or acceptance?
Will I be able to submit a study and not respond or address HRPP/IRB feedback?
How can I get in contact with HRPP/IRB with any questions or concerns?
What if I don’t know if my project requires HRPP/IRB review?
If you don’t know whether review is necessary, the HRPP webpage contains resources about whether a study is human subjects research and self-determination tools.
If after reviewing this information you are still questioning whether review is necessary, then reach out to HRPP prior to completing an application. We are happy to help!
How long does it take for my study to receive IRB/HRPP approval or acceptance?
Review times are available on our HRPP/IRB Home Page and turnaround times are available on our QA/QI webpage. Turnaround times strongly depend on how quickly the research team can respond to feedback/requests from HRPP/IRB.
Once a submission has received a pre-review, the PI has 45 calendar days to bring the submission to approvable status or it will be withdrawn by HRPP. Once withdrawn the PI should incorporate HRPP feedback and resubmit if they still require approval.
Will I be able to submit a study and not respond or address HRPP/IRB feedback?
It is the PI’s responsibility to read emails and respond to feedback provided by HRPP. We do not follow up with PI’s during intake, but do follow up during HRPP/IRB reviews.
Once a submission has received a pre-review, the PI has 45 calendar days to bring the submission to approvable status or it will be withdrawn by HRPP. Once withdrawn the PI should incorporate HRPP feedback and resubmit if they still require approval.
It is the PI’s responsibility to inform HRPP if they have any response delays during the review process.
What if I have sponsored funding that may impact when I need to receive IRB/HRPP approval or acceptance by?
Inform HRPP if you have any sponsored funding deadlines for receiving IRB/HRPP approval or acceptance. It is recommended you mention this deadline in your submission through a Huron Comment with documentation, so that HRPP can properly prioritize the review.
How can I get in contact with HRPP/IRB with any questions or concerns?
♦
We have many ways to contact us: email, phone, Zoom, TEA Time, Meeting Requests. Contacts and links below. Just reach out, we are happy to help!
General Email: [email protected]
General Phone: (401) 863-3050






