Looking for REVIEW TIMES?
The status of New Submissions and Revisions are available in Huron. Please review the workflow to see the state of these submissions and any potential feedback. Current submission dates will be updated weekly on Fridays.
HRPP response times may be delayed due to high submission volume and reduced staffing, we appreciate your patience. HRPP is currently reviewing submissions entered into the queue as of:
New Submissions (STUDY)
|
March 6th
|
Revisions (MOD)
|
March 6th
|
Undergraduate Applications
|
April 26th
|
View HRPP/IRB Metrics including submission volume and turnaround times by review type.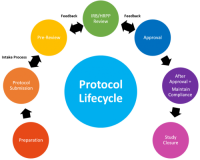
For a detailed description of the IRB/HRPP process for protocol management, visit the Lifecycle of a Submission webpage.
******************************************************

Did you know we have an advisory group? The Brown Human Research Advisory Group (BHRAG) is a valued partner to the HRPP - Find out how to know & grow with us!
******************************************************
For additional reference for planning and submission purposes, please visit the QA/QI page for information concerning HRPP metrics. This information is updated on a monthly basis in order to provide the most recent information regarding turn-around-times of all types of submissions as well as overall program volume.
Full Board Meeting Dates & Submission Deadlines
Submissions received by the submission deadline will be considered for review at the corresponding monthly IRB meeting. More information on meeting and deadline dates can be found here or viewed on the calendar below.
|
Brown's FWA #: 00004460 |
Brown's IRB #: 00000556 |
|
IRB Chair: |
IRB Vice Chair: |
| IRB Membership | |
IRB / HRPP Calendar
Click the  icon in the lower right corner to add us to your Google calendar!
icon in the lower right corner to add us to your Google calendar!

