The IACUC Review Process
Jump To:
Expectations
It is the PI’s responsibility to adhere to all deadlines, policies, and regulations and to respond to comments during protocol review in a timely manner. The IACUC review process cannot be expedited or modified per federal regulations (NOT-02-0064),(PHS Policy Section IV.C.2). Please plan ahead, submit early, and allow adequate time for processing. Note that all submissions that remain in the same status during review for more than 45 days will be deleted to maintain the integrity of the review. To review the IACUC meeting dates and deadlines, click HERE!
Note for COVID-19: Regulatory officials have repeatedly affirmed that there is NO flexibility in expiration dates or deadlines despite the pandemic. Please plan accordingly to ensure that you meet all deadlines.
Submission Review Time Averages*
|
|
|
New/de novo protocol |
60 days |
|
Substantive Amendment |
26 days |
|
Administrative Change/Personnel Removal |
1-2 days |
|
Annual Continuation |
22 days |
*Review times are time from receipt in the ARC office to IACUC approval and are based on actual 2019-2021 review data. These averages are intended as a guideline only and should not be construed in any way as a guarantee of review time. You can reduce these review times by an average of 40% by following a few simple Helpful Tips.
IACUC Review Process
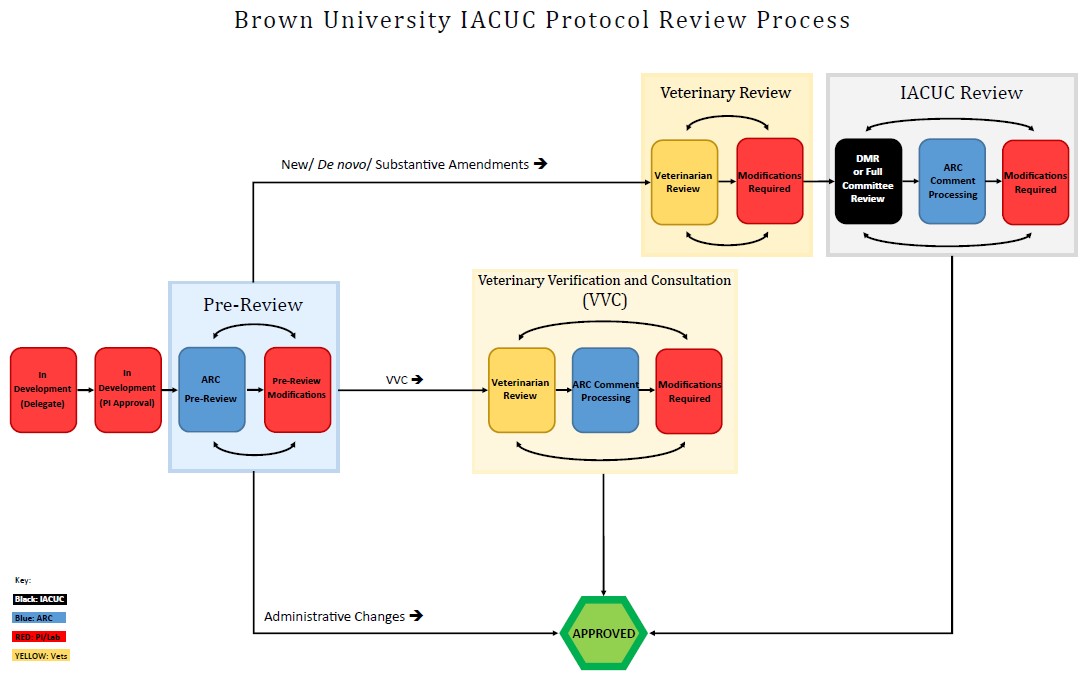
All live vertebrate animal research must be approved by the IACUC before any work may be done. The review process at Brown University includes 2-3 stages of review prior to approval.
Protocol Development: Once a submission is created in the system, it is considered “In Development” until it is submitted to the ARC team for Pre-Review.
ARC Pre-Review: All protocols are first reviewed by the ARC team for completeness. Please see the Example Protocol (login required) to get an idea of what the team will be looking for. The team will make comments and return the protocol to you for modifications as many times as necessary until it is complete.
Veterinary Review: A Brown University veterinarian may then review the protocol for appropriate animal use, drug doses, housing and enrichment, etc. The veterinarian may make comments at this stage and return the protocol directly to you for modifications as many times as necessary until he/she is satisfied.
IACUC Review: All protocols except Substantive Amendments that qualify for VVC (see below) and Administrative Changes must be reviewed by the IACUC.
-
Designated Member Review (DMR): Most protocols are reviewed by one or two delegated content expert members of the IACUC via Designated Member Review (DMR). In this case, the DMR reviewers will make comments and the protocol will be returned to you for modifications as many times as necessary until the reviewers are satisfied. To protect the integrity of review, the identity of the DMR reviewers assigned to your protocol is kept confidential. Comments will be sent to you by the ARC team.
-
Full Committee Review: Certain procedures and protocols requiring more oversight may require Full Committee (FCR) at a convened monthly IACUC meeting. The following are examples of common reasons a protocol may be called to FCR:
- Unrelieved painful and/or distressful procedures;
- Use of Non-Human Primates;
- Protocols involving a principal investigator (or student mentee) who has not previously submitted a protocol to the Brown IACUC;
Note, however, that these are just guidelines. Any IACUC member may request FCR of any protocol at any time (PHS Policy Section IV.C.2). A follow-up communication will be sent to you after the committee meeting regarding your protocol and the next steps as determined by the committee. If you are notified that your protocol will be reviewed by the full committee, please see IACUC Meetings and Information for dates and deadlines.
Helpful Tips to Ensure a Smooth Review:
-
Respond to comments immediately. 40% of all review time is spent in modifications with the lab. You can substantially reduce this by watching your InfoEd “Things to Do” inbox and responding to comments as soon as they come in. Please note that submissions remaining in the same status during review for more than 45 days will be deleted to maintain the integrity of the review.
-
Consult with a CARE veterinarian prior to submitting a protocol or amendment with a satellite facility or Category D/Category E or potentially painful/ distressful procedures. See below for applicable policies:
-
Obtain IBC (Biosafety), RSC (Radiation), or ESCRO (Stem Cell) Committee approval prior to submitting a protocol or amendment with hazardous (biological OR chemical) or radioactive agents or stem cell use. The IACUC protocol CANNOT be approved without approval letters from these committees as applicable. Note that this can take 30 days or more, so plan ahead!
-
Fill out all fields completely. This will reduce the amount of minor errors that are sent back for revision at pre-review. See the Example Protocol (login required) for how to fill out the forms
-
Make sure your Experimental Design and Animal Numbers match. The experimental groups and groups listed in the animal numbers section must match.
-
Choose the correct submission template at the start, otherwise you must start completely over. If you create a Continuation but want to submit an Amendment, for example, the submission cannot be processed. If you need help creating the correct submission template, contact [email protected]
-
Use Chrome as your internet browser. Some site features do not work properly in Firefox, Safari, or Edge.
-
Build flexibility into your protocol: provide ranges for volumes, needles, time points, and numbers when appropriate. This reduces administrative burden by reducing the need for amendments.
-
For information on how to submit something in InfoEd, click HERE!
